
Deepak Sapra
CEO, Pharmaceutical Services
And Active Ingredients

Gunjan Singh
Vice President and
Head- US & Europe

Sumit Kumar
Vice President and
Region Head - US

B Vivek
Executive VP and Global Head Supply,
Demand Planning and Delivery

Kalpesh Kumar Modi
Vice President and
Region Head - Europe

Puneet Rastogi
Associate VP - US

Pratik Singh Bais
Director, Mexico &
Central America

Juli Asmonga
Associate VP - US
Our Team at
DCAT 2023
Our Team at
DCAT 2023

Deepak Sapra
CEO, Pharmaceutical Services
And Active Ingredients

Gunjan Singh
Vice President and
Head- US & Europe

Sumit Kumar
Vice President and
Region Head - US

B Vivek
Executive VP and Global Head Supply,
Demand Planning and Delivery

Kalpesh Kumar Modi
Vice President and
Region Head - Europe

Puneet Rastogi
Associate VP - US

Pratik Singh Bais
Director, Mexico &
Central America

Juli Asmonga
Associate VP - US
About DCAT 2023

DCAT Week 2023 is the premier global event for companies engaged in the bio/pharmaceutical manufacturing value chain. Hosted by the Drug, Chemical & Associated Technologies Association (DCAT), this annual event brings together innovator and generic drug manufacturers, suppliers of ingredients, development and manufacturing services, and related technologies for four days of networking, education, and business development.
This year, DCAT Week will be held on March 20-23, 2023, in New York City. The event features a robust program of educational sessions and workshops and an exhibition floor showcasing the latest products, services, and technologies from leading companies in the industry.
At DCAT Week 2023, attendees will have the opportunity to connect with industry leaders and experts, learn about the latest trends and advancements in bio/pharmaceutical manufacturing, and explore new business opportunities. Whether you're a manufacturer, supplier, or service provider, DCAT Week is the perfect platform to showcase your company and connect with potential partners and customers.
In addition to the exhibition and educational sessions, DCAT Week 2023 also features networking events and receptions, providing attendees with ample opportunities to connect with their peers and expand their professional networks. Don't miss the chance to be a part of this premier industry event. Schedule your meeting today and join us at DCAT Week 2023 in New York City!
About Dr.Reddy’s API Business
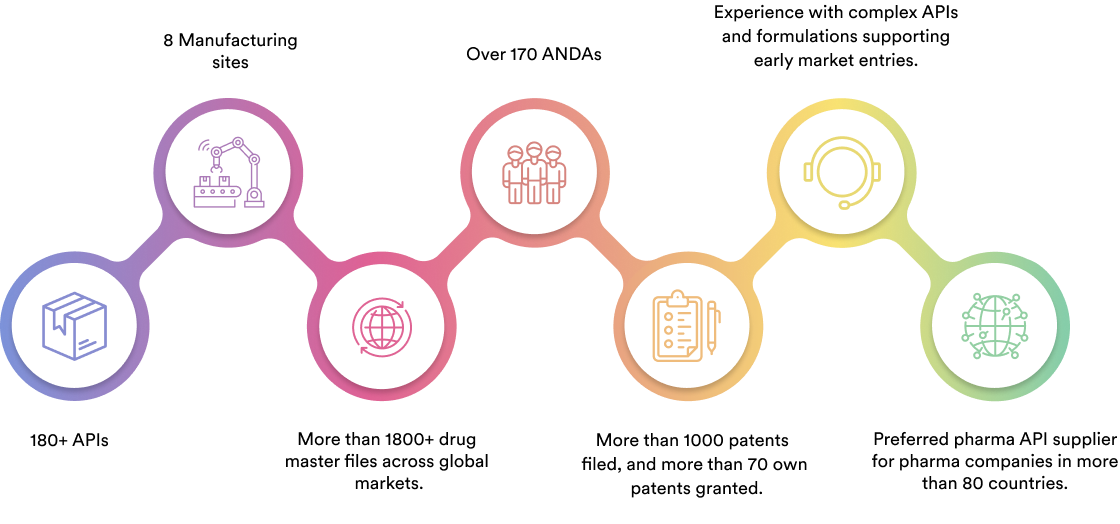
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
Our Achievement


Manufacturing Location

Renuncia
Ninguna información en este sitio web, incluyendo cualquier referencia a cualquier producto o servicio, constituye una oferta de venta ni se interpretará como tal. Los productos protegidos por patentes válidas no se ofrecen ni suministran para uso comercial. Sin embargo, en ciertos casos, a discreción exclusiva de Dr. Reddy's y sujeto a los requisitos legales locales, las cantidades de investigación de dichos productos pueden ofrecerse para fines de presentaciones regulatorias según la Sección 107A de la Ley de Patentes de la India (exención de Bolar), donde existan dichas exenciones regulatorias. Los compradores deben realizar su propia evaluación del producto o servicio, incluyendo el escenario de patentes en sus respectivos mercados, y serán responsables de todas las responsabilidades relacionadas con las patentes. Dr. Reddy's renuncia a todas las garantías, expresas o implícitas, incluyendo, entre otras, las garantías de comerciabilidad, idoneidad para un propósito particular y no infracción.




