
Nirav Shah
Vice President and
Region Head

Takayuki Hato
Regional Sales Lead,
Sales and Marketing

Masahiro Wakabayashi
Regional Sales Manager,
Sales and Marketing

Rohan kaushik
Associate VP, Business Development,
Sales and Marketing

Aditya Gudimella
Associate Director, Marketing
Our Team at
CPHI Japan
About CPHI Japan

CPHI Japan is the premier event for the pharmaceutical industry in Japan, providing a platform for international companies to connect and do business with key players in the Japanese market. In its 20th anniversary, the event is expected to attract over 200 exhibitors from across the entire pharmaceutical supply chain, showcasing the latest products, solutions, and innovations in the industry.
Attendees can expect to discover the latest products and services from ingredients, contract services, and biopharma to technology, packaging, and machinery. They will also have the opportunity to meet and network with industry leaders, search for potential partners, and arrange meetings with potential partners online through the event's chat service.
Whether you are a large multinational corporation or a small start-up, CPHI Japan provides the perfect platform to expand your horizons in the Japanese pharmaceutical market. Join us at the event and take advantage of the unique opportunities to connect and grow your business.
About Dr.Reddy’s API Business
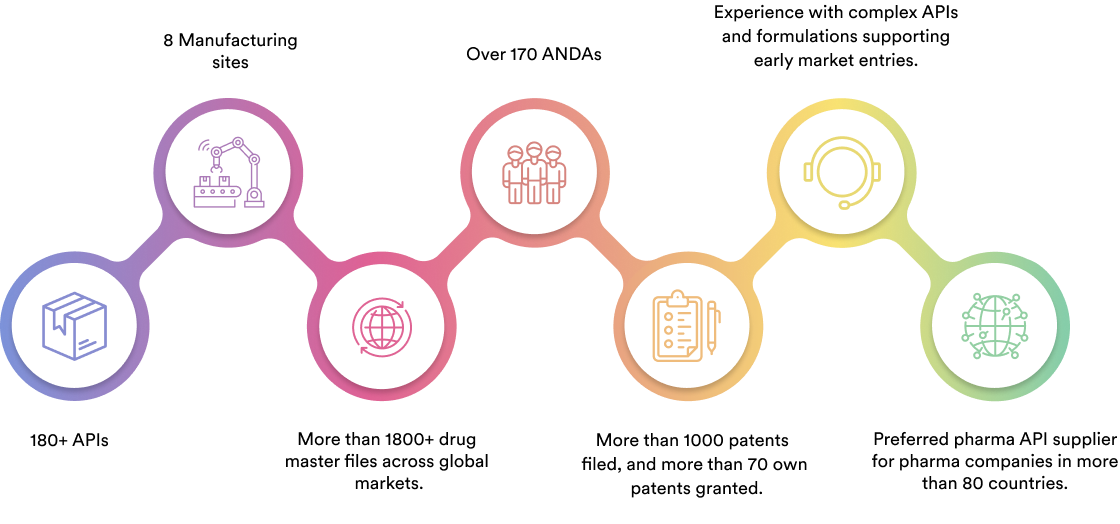
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
Our Achievement


Manufacturing Location

免責事項
このウェブサイト上のいかなる情報も、製品またはサービスへの言及を含め、販売の申し出を構成するものではなく、販売の申し出を表すものと解釈されるものでもありません。有効な特許により保護されている製品は、商用目的で提供または提供されるものではありません。ただし、特定のケースでは、Dr. Reddy の独自の裁量により、現地の法的要件に従って、そのような製品の研究用数量が、規制免除が存在する場所に、インド特許法第 107A 条 (Bolar 免除) に基づく規制提出の目的で提供される場合があります。購入者は、それぞれの市場における特許シナリオを含む製品またはサービスについて独自の評価を行う必要があり、すべての特許関連法的責任を負うことになります。Dr. Reddy は、商品性、特定目的への適合性、および非侵害の保証を含むがこれらに限定されない、明示または黙示を問わずすべての保証を否認します。




