
Deepak Sapra
CEO, Pharmaceutical Services
And Active Ingredients

Gunjan Singh
Vice President and
Head- US & Europe

Sumit Kumar
Vice President and
Region Head - US

B Vivek
Executive VP and Global Head Supply,
Demand Planning and Delivery

Kalpesh Kumar Modi
Vice President and
Region Head - Europe

Puneet Rastogi
Associate VP - US

Pratik Singh Bais
Director, Mexico &
Central America

Juli Asmonga
Associate VP - US
Our Team at
DCAT 2023
Our Team at
DCAT 2023

Deepak Sapra
CEO, Pharmaceutical Services
And Active Ingredients

Gunjan Singh
Vice President and
Head- US & Europe

Sumit Kumar
Vice President and
Region Head - US

B Vivek
Executive VP and Global Head Supply,
Demand Planning and Delivery

Kalpesh Kumar Modi
Vice President and
Region Head - Europe

Puneet Rastogi
Associate VP - US

Pratik Singh Bais
Director, Mexico &
Central America

Juli Asmonga
Associate VP - US
About DCAT 2023

DCAT Week 2023 is the premier global event for companies engaged in the bio/pharmaceutical manufacturing value chain. Hosted by the Drug, Chemical & Associated Technologies Association (DCAT), this annual event brings together innovator and generic drug manufacturers, suppliers of ingredients, development and manufacturing services, and related technologies for four days of networking, education, and business development.
This year, DCAT Week will be held on March 20-23, 2023, in New York City. The event features a robust program of educational sessions and workshops and an exhibition floor showcasing the latest products, services, and technologies from leading companies in the industry.
At DCAT Week 2023, attendees will have the opportunity to connect with industry leaders and experts, learn about the latest trends and advancements in bio/pharmaceutical manufacturing, and explore new business opportunities. Whether you're a manufacturer, supplier, or service provider, DCAT Week is the perfect platform to showcase your company and connect with potential partners and customers.
In addition to the exhibition and educational sessions, DCAT Week 2023 also features networking events and receptions, providing attendees with ample opportunities to connect with their peers and expand their professional networks. Don't miss the chance to be a part of this premier industry event. Schedule your meeting today and join us at DCAT Week 2023 in New York City!
About Dr.Reddy’s API Business
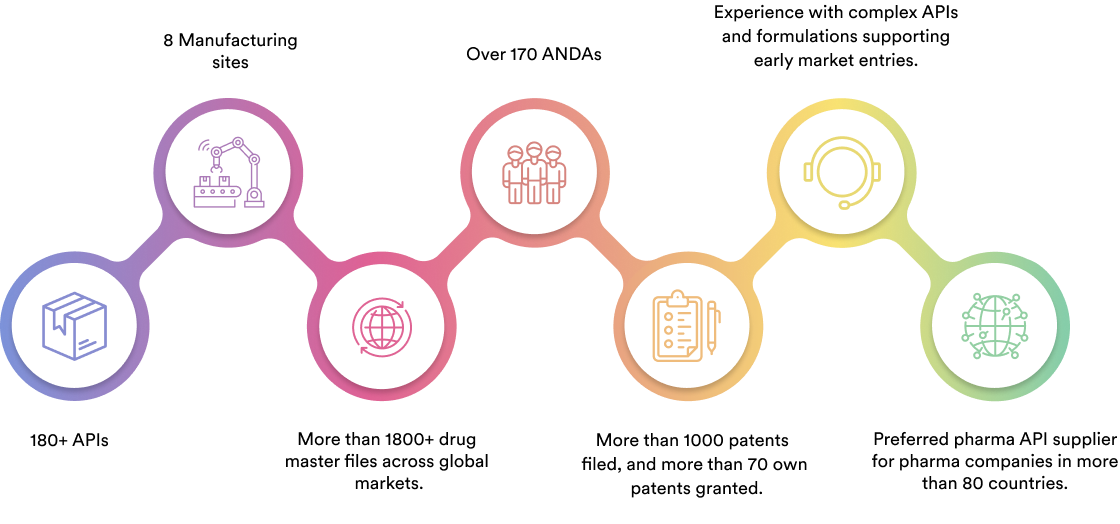
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
Our Achievement


Manufacturing Location

免責事項
このウェブサイト上のいかなる情報も、製品またはサービスへの言及を含め、販売の申し出を構成するものではなく、販売の申し出を表すものと解釈されるものでもありません。有効な特許により保護されている製品は、商用目的で提供または提供されるものではありません。ただし、特定のケースでは、Dr. Reddy の独自の裁量により、現地の法的要件に従って、そのような製品の研究用数量が、規制免除が存在する場所に、インド特許法第 107A 条 (Bolar 免除) に基づく規制提出の目的で提供される場合があります。購入者は、それぞれの市場における特許シナリオを含む製品またはサービスについて独自の評価を行う必要があり、すべての特許関連法的責任を負うことになります。Dr. Reddy は、商品性、特定目的への適合性、および非侵害の保証を含むがこれらに限定されない、明示または黙示を問わずすべての保証を否認します。




