
Kundan Reddy
Associate Director -
Sales and Marketing

Kapil Choudhury
Head of Portfolio strategy
and Region Head China

Tammy Tang
Director - Sales and Marketing

Ashwinikumar Kulkarni
Associate VP and Head,
Global Customer Support
and Program Management

Nirav K Shah
Vice President and Head -
North Asia

Durga Bhavani
Vertical Head -
Regulatory Affairs

Rakesh Varma
Director,
API Product Management

AVT Reddy
Vice President and
Head Chemical Sourcing

Aditya Gudimella
Associate Director, Marketing
Our Team at
CPHI China
About CPHI China

CPHI China is Asia's premier pharma event that brings together leading pharma experts from across the end-to-end pharma supply chain. The event offers a one-stop shop for the pharmaceutical industry, allowing attendees to access a vast array of pharmaceutical ingredients, machinery, laboratory equipment, environmental protection, pharma packaging, and processing solutions - all conveniently located under one roof.
With thousands of local and international exhibitors in attendance, this year's edition promises to be the best one yet. Attendees can source cutting-edge solutions, and attend a range of presentations, forums, showcases, and other industry events to expand their knowledge and expertise.
The event offers more flexibility and pre-connect opportunities to attendees who want to network with peers face-to-face to connect with potential business prospects and collaborate to overcome challenges. With a jam-packed conference planned across 3 days, attendees can learn from experts, leaders, and fresh new voices, staying ahead of all pharma trends.
Join us this June, where the show will return for the first time in three years, and be part of this exciting event that offers limitless possibilities for professionals looking to expand their business in China's booming pharma market!
About Dr.Reddy’s API Business
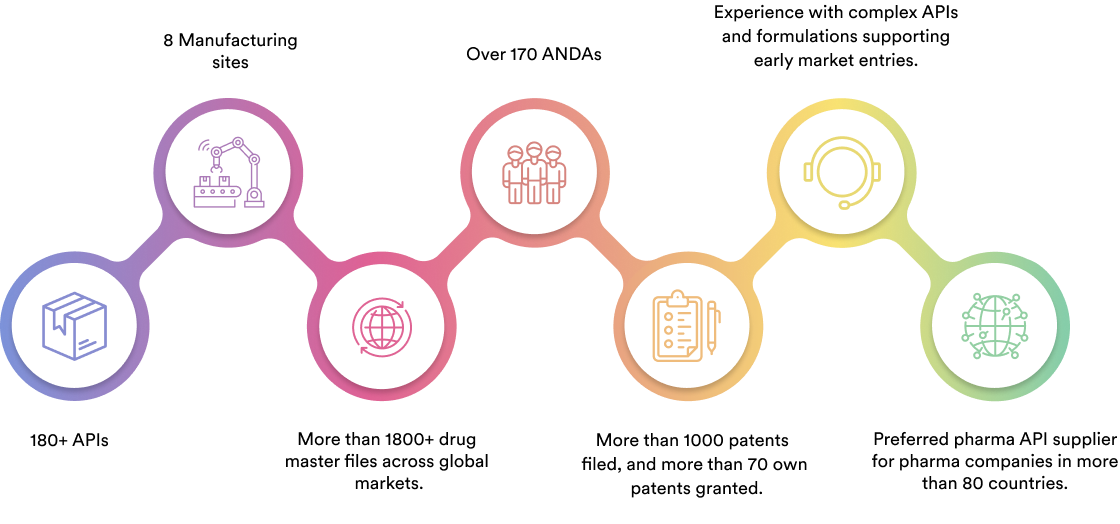
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
Our Achievement


Manufacturing Location

免责声明
本網站上的任何信息,包括對任何產品或服務的任何提及,均不構成銷售要約或被解釋為代表銷售要約。受有效專利保護的產品不得提供或供應用於商業用途。但是,在某些情況下,雷迪博士可自行決定並根據當地法律要求,在存在此類監管豁免的任何地方,提供此類產品的研究數量,以根據《印度專利法》第 107A 條(Bolar 豁免)進行監管提交。購買者應對其各自市場的產品或服務(包括專利情況)進行獨立評估,並對所有與專利相關的責任負責。 Dr. Reddy's 不承擔任何明示或暗示的保證,包括但不限於適銷性、適用於特定用途和非侵權的保證。




