Our Team at CPHI Japan 2025

Nirav Shah
Vice President, and Head, API - North Asia

Masahiro Wakabayashi
Director, API Sales & Marketing, Japan

Sriram Karthikeya Chavali
Associate Director, Business Development, Japan

Dr. Akihito Kitajima
Head, API Regulatory Affairs, Japan
About Dr.Reddy’s API Business
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 250+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
-
250+ APIs
-
8 Manufacturing sites
-
1950 drug master files across global markets.
-
Over 325 ANDAs
-
More than 1000 patents field, and more than 70 own patents granted
-
Experience with complex APIs and formulations supporting early market entries.
-
Preferred pharma API supplier for pharma companies in more than 80 countries.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
What we achieved in the past year:


Our Achievement (awards)

25/04/2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

05/04/2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

05/12/2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

04/11/2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

11/11/2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

19/10/2020
Dr. Reddy's recognized at the CPhI Pharma Awards 2020 as the winner in the category of ‘Excellence in Pharma: Sustainability’
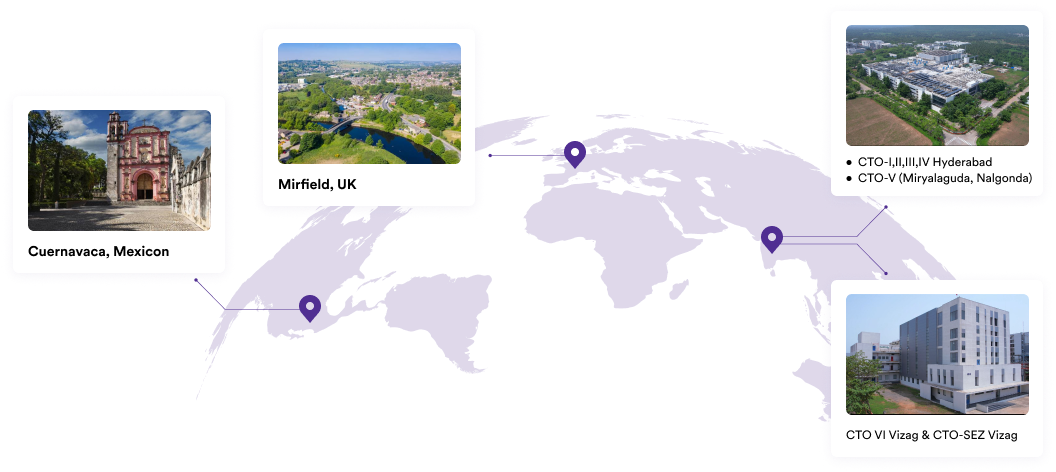
Our Plants
About CPHI Japan 2025
CPHI Japan 2025 is a leading event in the pharmaceutical industry, set to take place from April 9 to 11, 2025, at Tokyo Big Sight in Tokyo, Japan. Bringing together over 26,000 industry professionals and 730+ exhibitors from 60 countries, the event serves as a key platform for networking, collaboration, and knowledge-sharing. With a strong focus on innovation and business growth, CPHI Japan covers the entire pharmaceutical supply chain, offering insights into the latest trends, regulatory updates, and technological advancements shaping the industry.
Networking is at the heart of CPHI events, offering professionals a chance to build meaningful partnerships and strengthen existing collaborations. Dedicated networking lounges, informal meetups, and digital matchmaking tools help attendees connect with potential business partners, service providers, and key decision-makers. The event creates opportunities to explore new markets, establish supply chain relationships, and stay informed about the latest innovations driving the pharmaceutical sector forward. Join us to discover our innovative solutions, and learn how we can contribute to the advancement of the pharmaceutical sector together

Disclaimer
No information on this website, including any reference to any product or service constitutes an offer for sale or be construed as representing an offer for sale. Products protected under valid patents are not offered or supplied for commercial use. However, in certain cases, at Dr. Reddy's sole discretion, and subject to local legal requirement, the research quantities of such products may be offered for the purpose of regulatory submissions under Section 107A of the Indian Patent Act (Bolar exemption), wherever such regulatory exemptions exist. The buyers should make their independent evaluation of the product or service including, patent scenario in their respective markets and will be responsible for all patent related liabilities Dr. Reddy's disclaims all warranties, express or implied, including but not limited to warranties of merchantability, fitness for a particular purpose and non-infringement.
Let’s discuss API sourcing

Join us at Hall - 9.1 | Booth - B48






