Our Team at DCAT Week 2025

Deepak Sapra
CEO, Pharmaceutical Services & Active Ingredients

Kalpesh Modi
Vice President, and Head – EU Market, API & B2B Collaborations

Dr. NM Sekhar
Vice President, API R&D

E Sreedhar
Executive Vice President, API Regulatory Affairs

Juli Asmonga
Associate Vice President, API Sales & Marketing - North America

Lalitha Kumari
Head API Product Management and Regional Sales Lead - US & Canada

Dileep Kumar K
Director, API Sales & Marketing - North America
About Dr.Reddy’s API Business
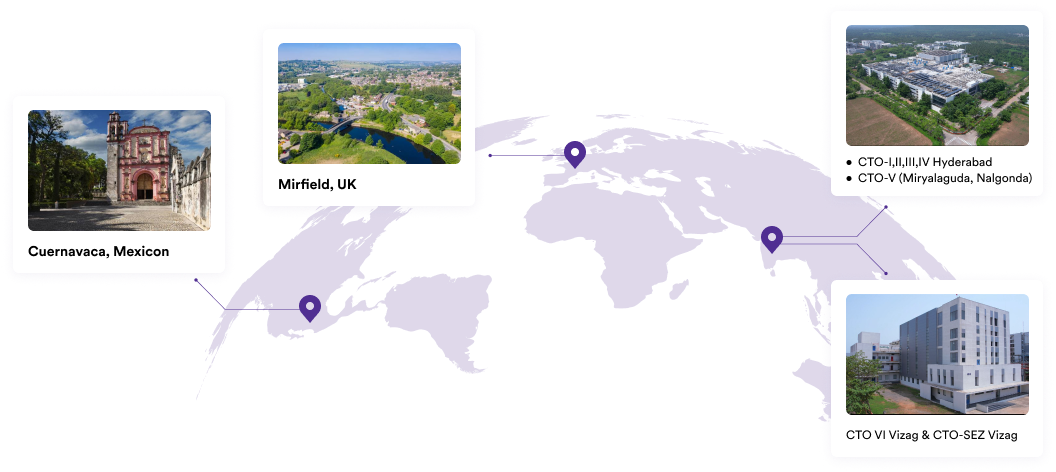
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 250+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
-
250+ APIs
-
8 Manufacturing sites
-
1900 drug master files across global markets.
-
Over 170 ANDAs and 500 DMFs
-
More than 1000 patents field, and more than 70 own patents granted
-
Experience with complex APIs and formulations supporting early market entries.
-
Preferred pharma API supplier for pharma companies in more than 80 countries.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
What we achieved in the past year:


Our Achievement (awards)

25/04/2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

05/04/2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

05/12/2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

04/11/2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

11/11/2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

19/10/2020
Dr. Reddy's recognized at the CPhI Pharma Awards 2020 as the winner in the category of ‘Excellence in Pharma: Sustainability’
Our Plants

About DCAT Week 2025
DCAT Week, hosted annually in New York City by the Drug, Chemical & Associated Technologies Association (DCAT), is the premier gathering for companies engaged in the global bio/pharmaceutical manufacturing value chain. It brings together over 10,000 industry professionals from across the world to foster collaboration, drive strategic business development, and shape the future of the bio/pharmaceutical industry.
DCAT is a not-for-profit, volunteer-led organization committed to the growth and advancement of its global membership. Joining DCAT and participating in DCAT Week isn’t just an event—it’s an investment in your company’s future within this dynamic and evolving industry. DCAT Week attracts key decision-makers from across the bio/pharmaceutical value chain, including professionals in sourcing, procurement, business development, corporate support, and licensing. The event serves as a hub for leaders looking to strengthen relationships, explore new business opportunities, showcase capabilities, and drive strategic objectives.
免责声明
本網站上的任何信息,包括對任何產品或服務的任何提及,均不構成銷售要約或被解釋為代表銷售要約。受有效專利保護的產品不得提供或供應用於商業用途。但是,在某些情況下,雷迪博士可自行決定並根據當地法律要求,在存在此類監管豁免的任何地方,提供此類產品的研究數量,以根據《印度專利法》第 107A 條(Bolar 豁免)進行監管提交。購買者應對其各自市場的產品或服務(包括專利情況)進行獨立評估,並對所有與專利相關的責任負責。 Dr. Reddy's 不承擔任何明示或暗示的保證,包括但不限於適銷性、適用於特定用途和非侵權的保證。
Let’s discuss API sourcing

Join us at Hall - 9.1 | Booth - B48






