Our Team at MAGHREB PHARMA Expo 2023
-

Netraja
Associate Director , Sales and Marketing - Turkey , Africa
-

Karun Gaur
Vice President - SEMEA
-

Namrata
Vice President - HR
About MAGHREB PHARMA Expo 2023
MAGHREB PHARMA Expo 2023 is the premier African pharmaceutical industry event. For the past 9 years, the expo has established itself as the go-to platform for pharmaceutical industry professionals in the region.

The expo brings together a diverse range of exhibitors showcasing the latest innovations in the field including pharmaceutical ingredients, cutting-edge production lines, advanced packaging solutions, state-of-the-art clean rooms, treatment systems for air, water production, sterile lines, biotech reactors, laboratory equipment, and quality control technologies.
With over 2,600 decision-makers in attendance from Algeria, Morocco, Tunisia, Mali, Mauritania, Libya, and beyond, the expo provides a unique opportunity for exhibitors to connect with key players in the industry and showcase their products and services to a highly engaged and qualified audience.
In addition to the exhibition, the expo also features a comprehensive conference program, including "learnshop" sessions where industry experts will be sharing their insights and knowledge on the latest developments in the pharmaceutical industry. These sessions are designed to provide valuable information and offer opportunities to network with other professionals in the field.
We look forward to meeting you at MAGHREB PHARMA Expo 2023, an event that is sure to inspire, inform and engage industry professionals. Come and be a part of the conversation shaping the future of the pharmaceutical industry in Africa.
About Dr.Reddy’s API Business
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
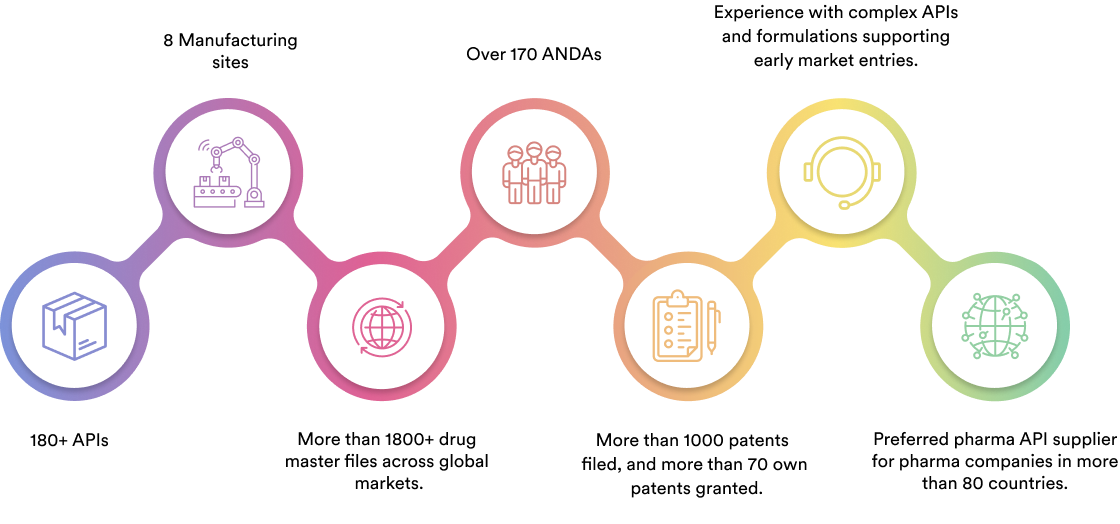
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
Sustainability
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.

Our Achievements

07/10/2021
The winner of the Best Indian Company in the US - Manufacturing Sector for the year 2021


03/11/2020
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2020

Our plants

免责声明
本網站上的任何信息,包括對任何產品或服務的任何提及,均不構成銷售要約或被解釋為代表銷售要約。受有效專利保護的產品不得提供或供應用於商業用途。但是,在某些情況下,雷迪博士可自行決定並根據當地法律要求,在存在此類監管豁免的任何地方,提供此類產品的研究數量,以根據《印度專利法》第 107A 條(Bolar 豁免)進行監管提交。購買者應對其各自市場的產品或服務(包括專利情況)進行獨立評估,並對所有與專利相關的責任負責。 Dr. Reddy's 不承擔任何明示或暗示的保證,包括但不限於適銷性、適用於特定用途和非侵權的保證。

