
资源
通过我们丰富的产品组合搜索高质量的活性药物成分。
White Paper

Dec 14, 2025
Analytical, Manufacturing, and Quality Attributes of Clopidogrel API: A Technical Overview
Nov 28, 2025
Siponimod Hemifumarate API - Technical Profile & Offerings

Oct 24, 2025
Palbociclib API - Technical Profile & Offerings

Sep 25, 2025
Tech Sheet on LCZ696 (Sacubitril-Valsartan) API

Aug 28, 2025
Dabigatran – Ready to fill Pellets

Aug 4, 2025
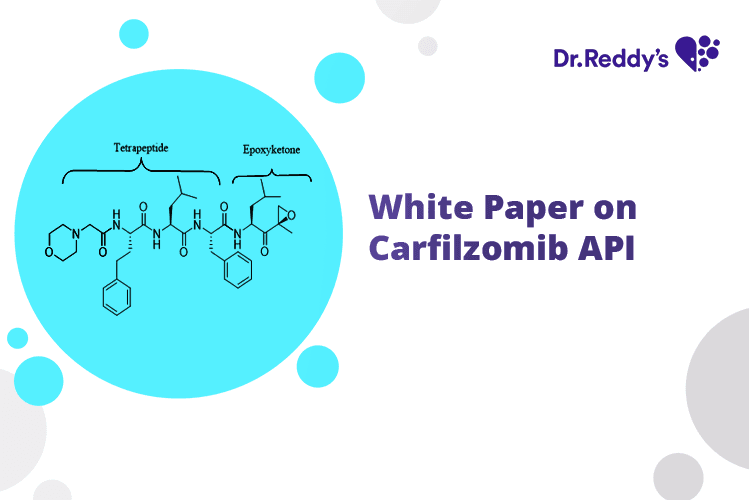
Carfilzomib: API Technical Profile & Offerings

Jul 15, 2025
Apremilast API: Comprehensive Technical Data Sheet and Offerings

Jun 16, 2025
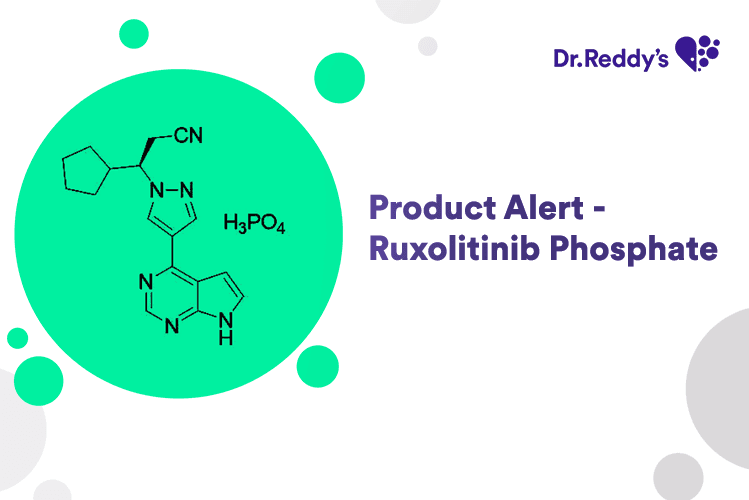
New Product Alert – Ruxolitinib Phosphate
May 26, 2025
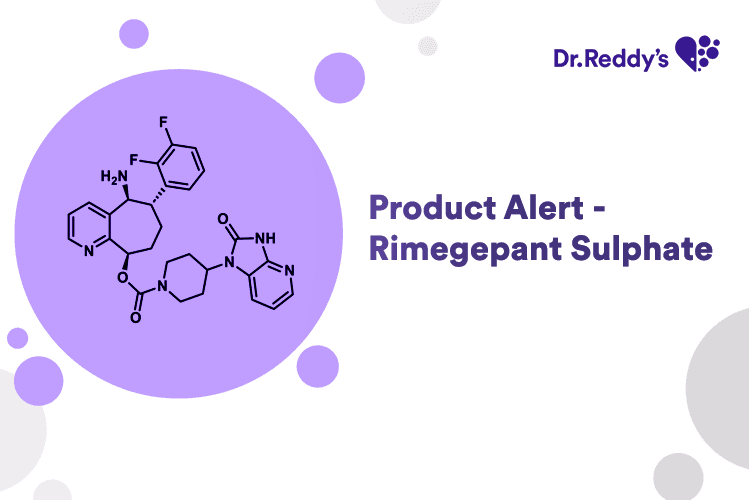
Product Alert - Rimegepant
May 5, 2025
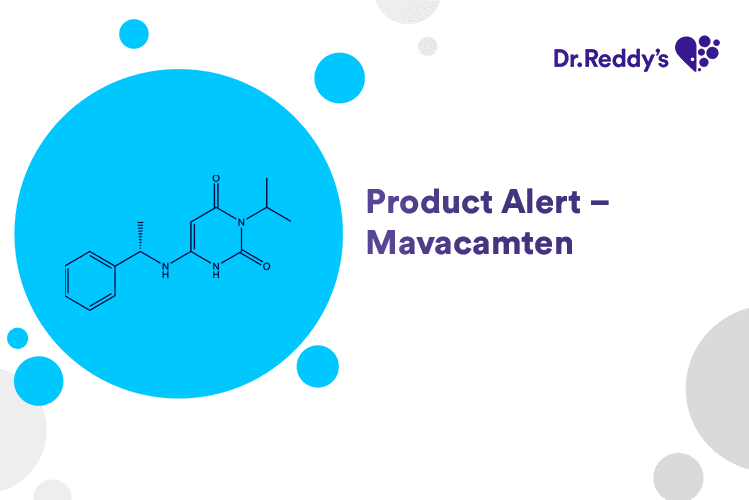
Product Alert – Mavacamten
Apr 24, 2025
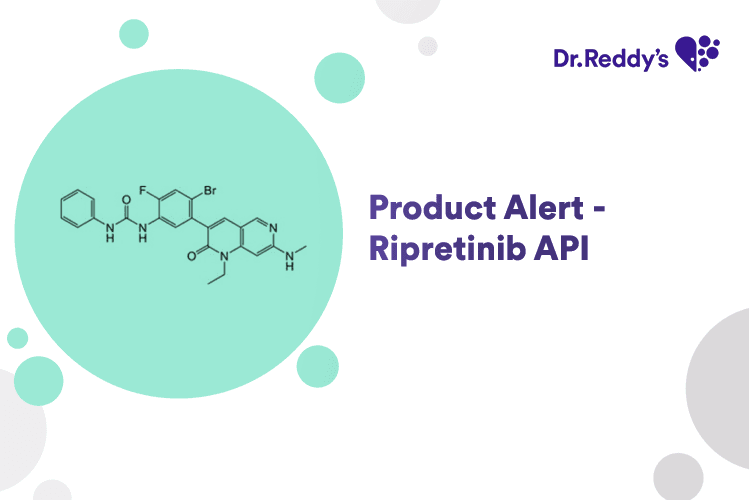
New Product Alert - Ripretinib
Mar 4, 2025
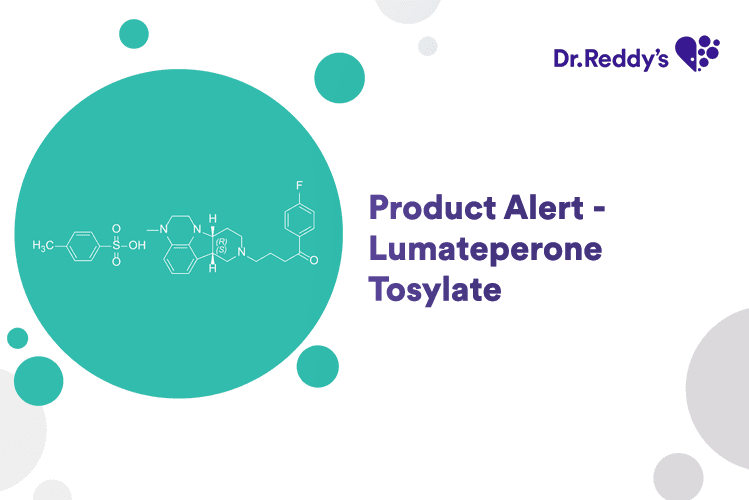
Product Alert - Lumateperone Tosylate
Feb 12, 2025
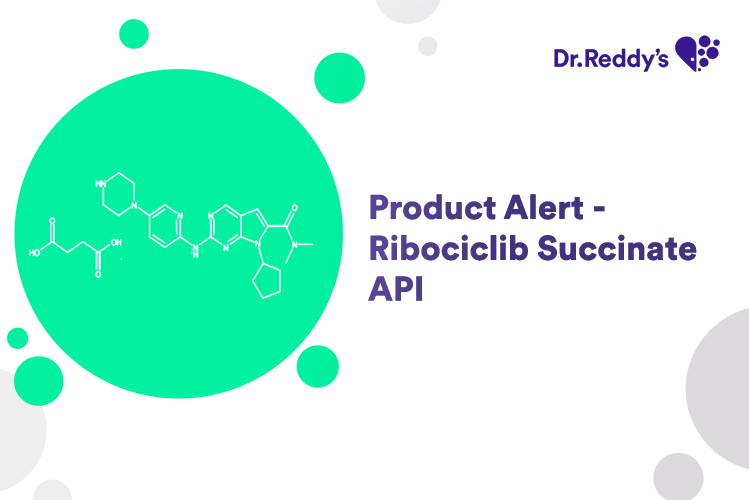
New Product Alert - Ribociclib
Jan 10, 2025
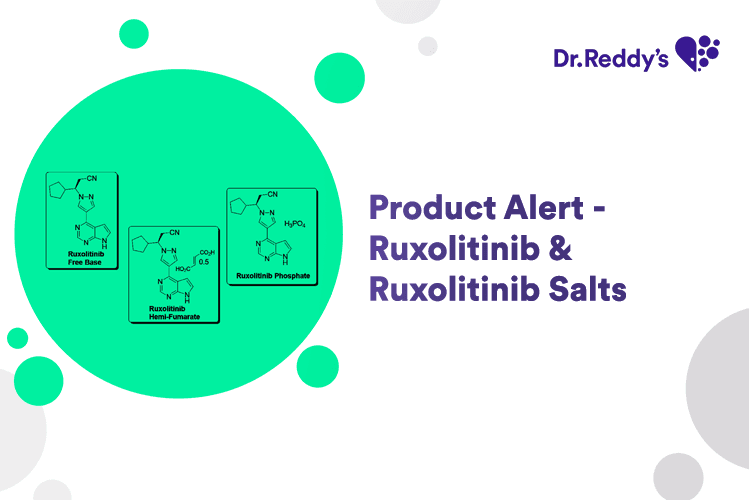
New Product Alert - Ruxolitinib & Salts

Dec 18, 2024
Tech Sheet On Nilotinib Hydrochloride

Oct 18, 2024
Tech Sheet – Mirabegron API

Sep 25, 2024
Tech sheet – Apremilast
May 31, 2024
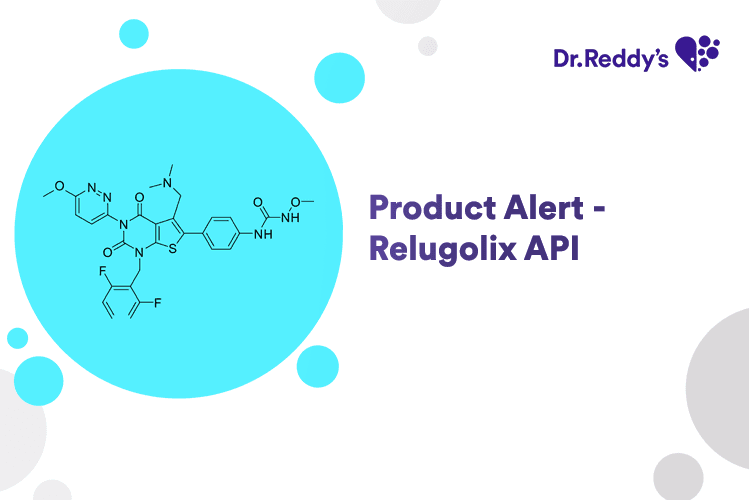
Product Alert - Relugolix API
May 31, 2024
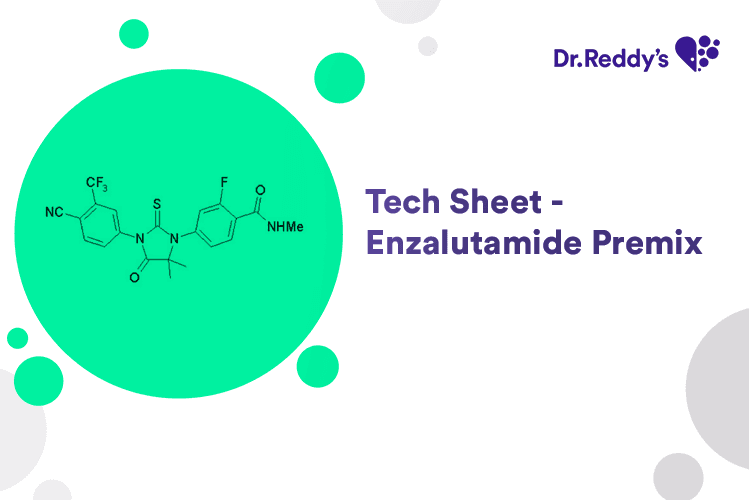
Tech Sheet on Enzalutamide Premix
May 31, 2024
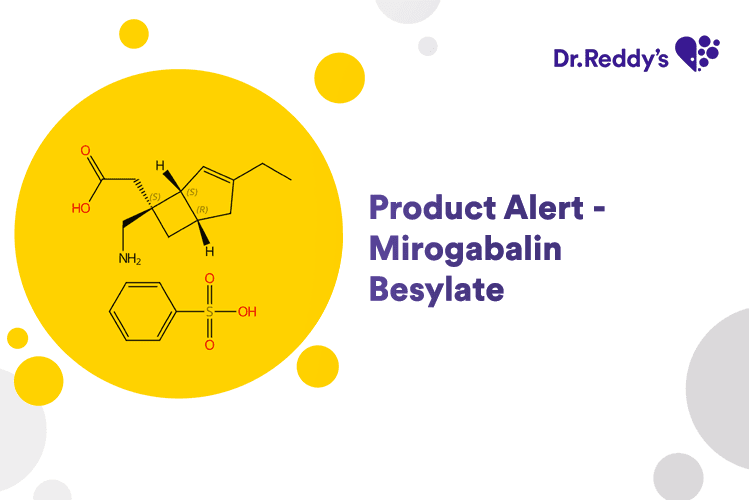
Product Alert – Mirogabalin Besylate
Apr 22, 2024
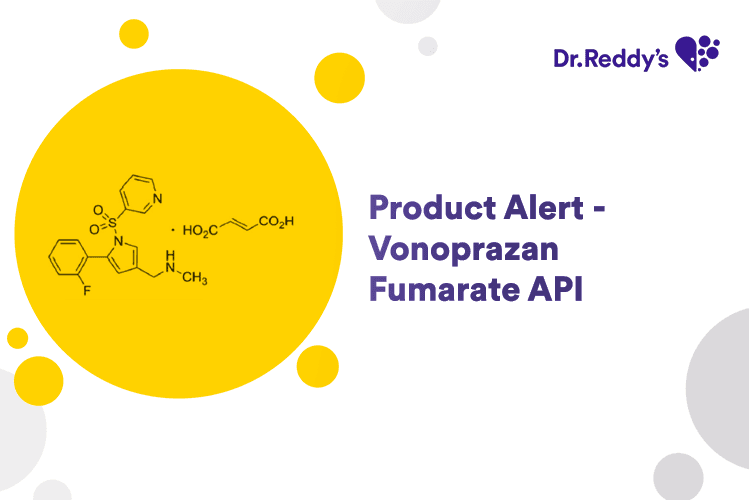
Product Alert – Vonoprazan Fumarate
Feb 21, 2024
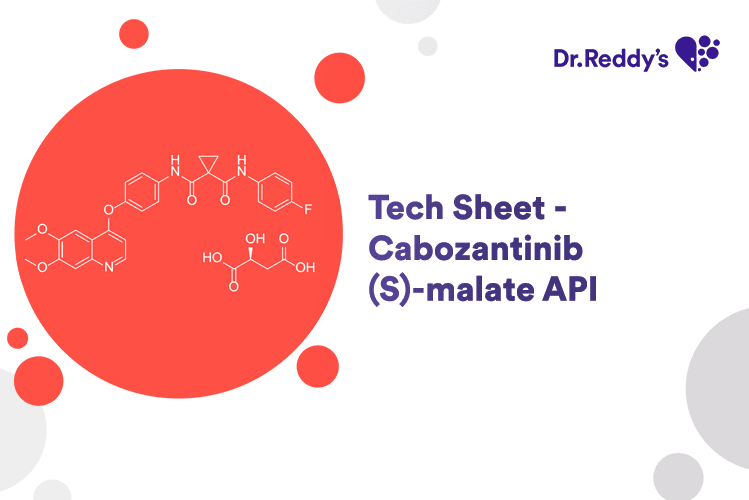
Tech Sheet - Cabozantinib (S)-malate API
Dec 7, 2023
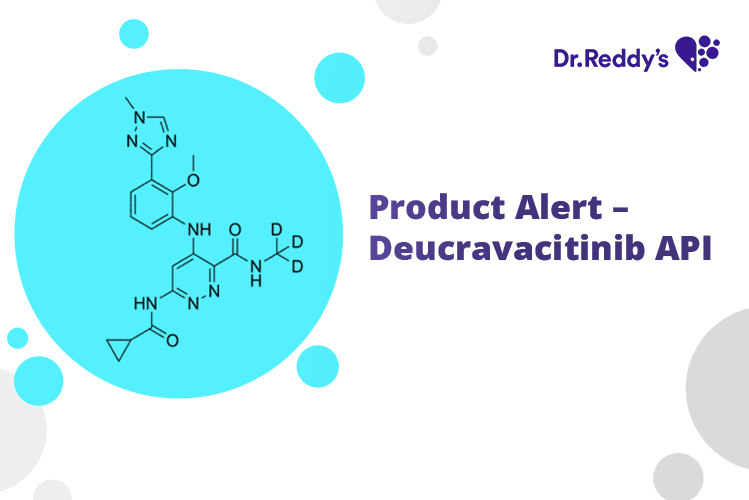
Product Alert – Deucravacitinib API
Dec 4, 2023
Substantially Pure Carfilzomib Amorphous for Generic Launch
Nov 6, 2023
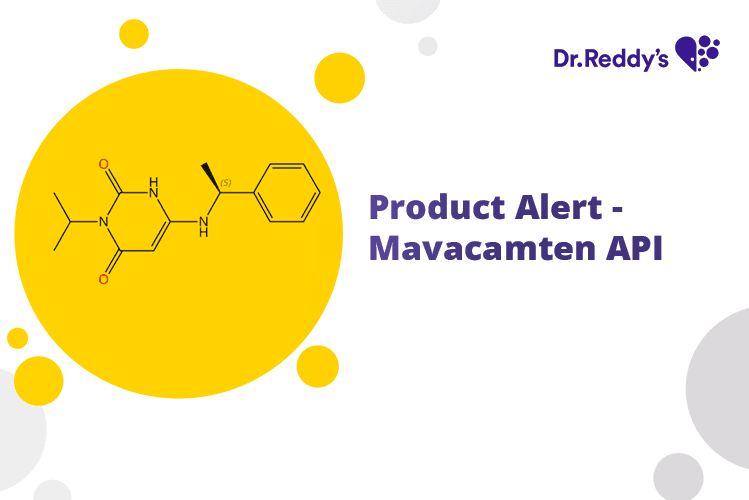
Product Alert – Mavacamten API
Jul 21, 2023
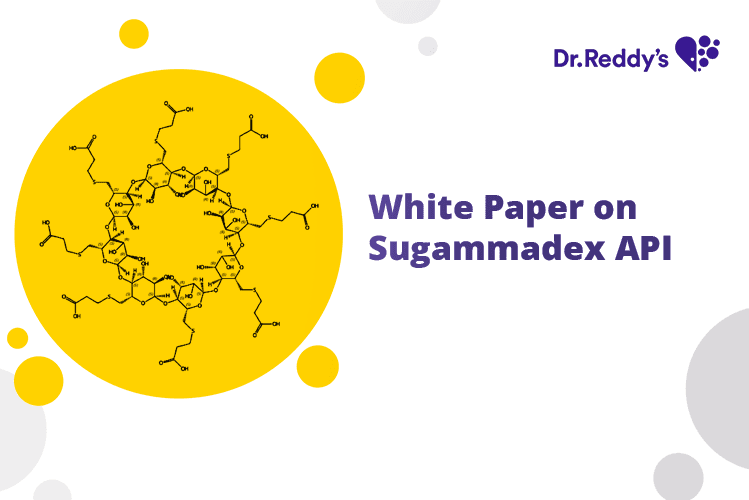
White Paper on Sugammadex API
Jul 21, 2023
Tech Sheet on Pregabalin API
Mar 20, 2023
Product Alert – Niraparib API
Mar 13, 2023
Product Alert – Tucatinib API
Mar 6, 2023
Product Alert – Pazopanib API
Jan 20, 2023
Tech Sheet on Midostaurin API
Jan 9, 2023
Tech Sheet on Tofacitinib Citrate
Nov 4, 2022
Tech Sheet on Dutasteride
Oct 14, 2022
Tech Sheet on Linagliptin
Oct 12, 2022
Tech Sheet on Voriconazole
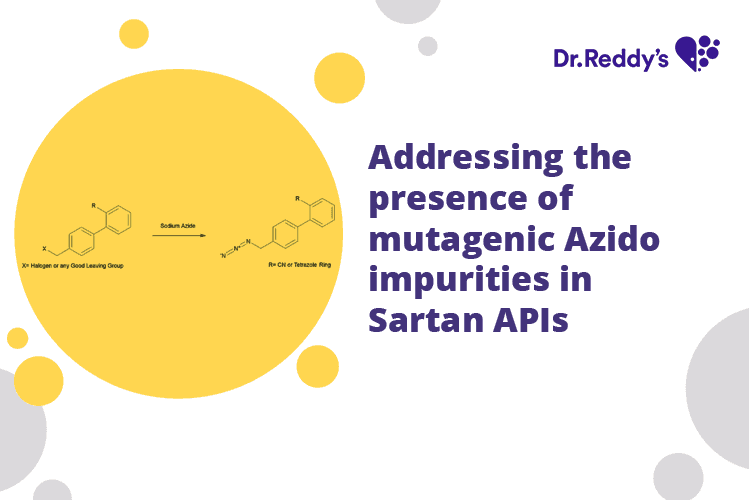
Aug 4, 2022
Addressing the presence of mutagenic Azido impurities in Sartan APIs
Aug 3, 2022
Co-Crystal and Customized Particle Size for Early Launch Opportunity of Siponimod API
Jul 29, 2022
New Product Alert – Lumateperone Tosylate API
Jun 15, 2022
Tech sheet: Dr. Reddy's Bempedoic Acid API Offerings
Apr 4, 2022
Tech sheet: Dr. Reddy's Olaparib API Offerings
Mar 30, 2022
Tech sheet: Dr. Reddy's Enzalutamide API Offerings
Feb 22, 2022
Tech Sheet - Dr. Reddy’s Eribulin Mesylate API offerings
Feb 9, 2022
Tech Sheet – Dr. Reddy’s Fexofenadine API offerings
Feb 4, 2022
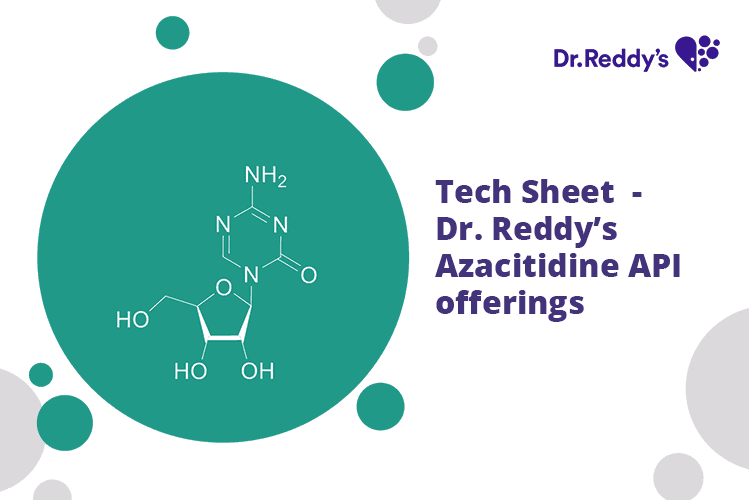
Tech Sheet - Dr. Reddy’s Azacitidine API offerings
Feb 4, 2022
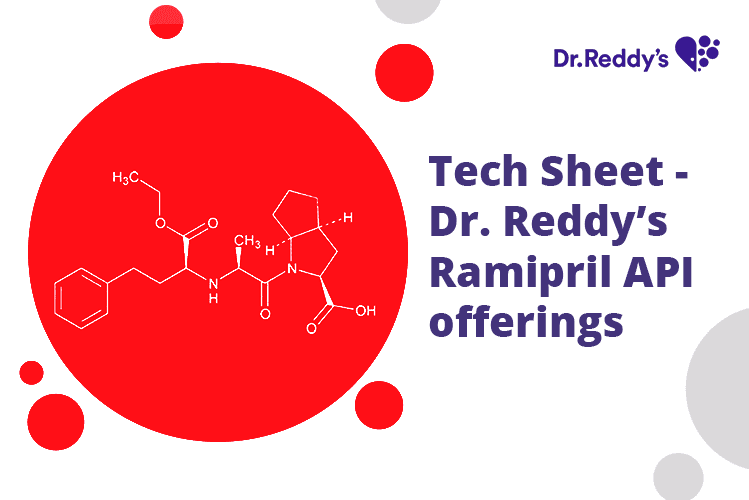
Tech Sheet - Dr. Reddy’s Ramipril API offerings
Dec 17, 2021
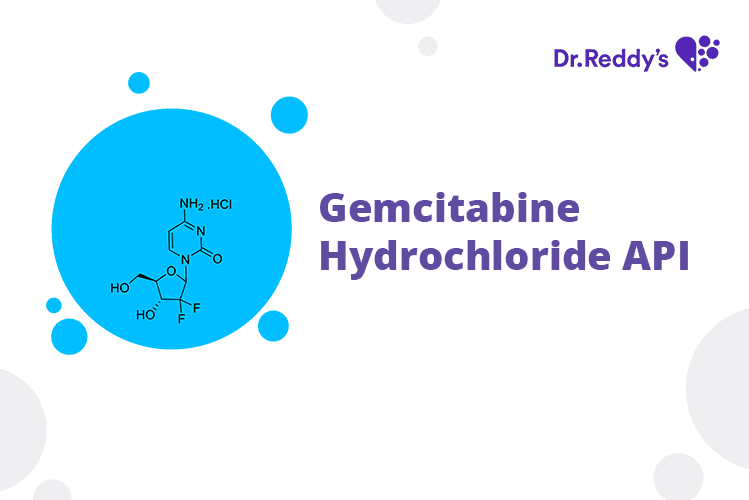
Tech Sheet - Dr. Reddy’s Gemcitabine API offerings
Dec 2, 2021
Tech Sheet: Dr. Reddy’s Abiraterone Acetate API offerings
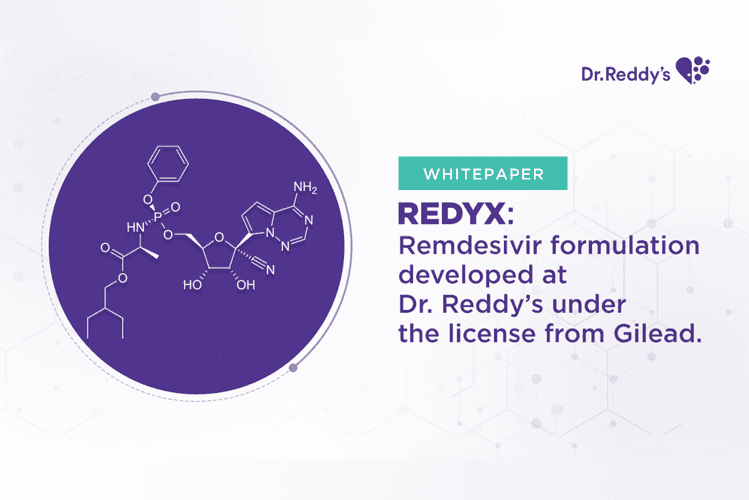
Oct 8, 2021
瑞德西韦(Remdesivir)系由瑞迪博士(Dr.Reddy’s)在吉利德(Gilead)许可下研发:

Aug 17, 2021
白皮书: 瑞迪博士的2 DG - 一种对住院的新冠病毒病患者来极具前景的治疗方法
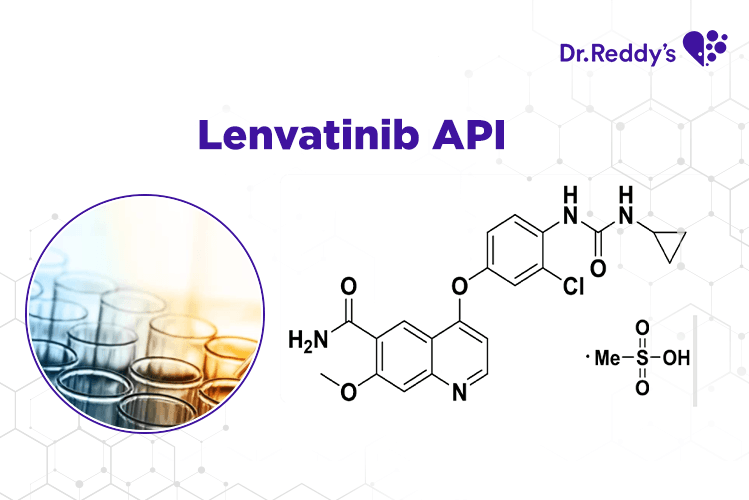
Aug 17, 2021
技术表: 瑞迪博士的乐伐替尼(Lenvatinib)原料药产品
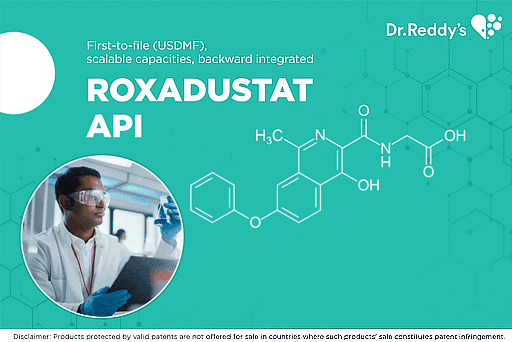
Jun 18, 2021
罗沙司他(ROXADUSTAT)- 多管齐下的发展战略
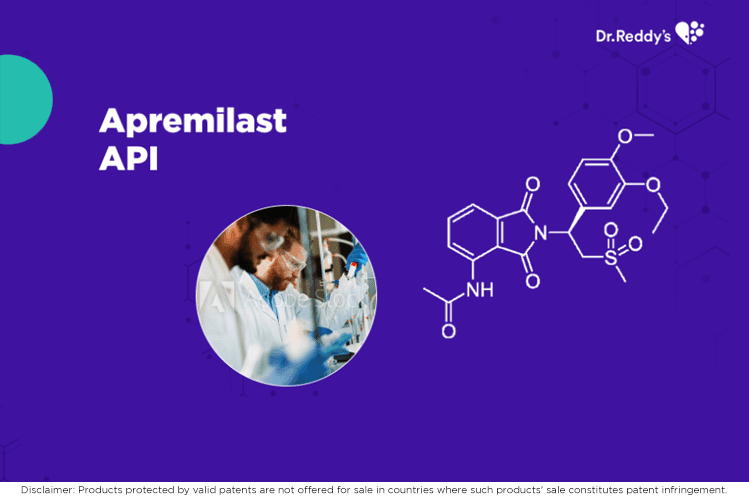
May 19, 2021
瑞迪博士的阿普斯特片(Apremilast)原料药和成品配方产品。
May 3, 2021
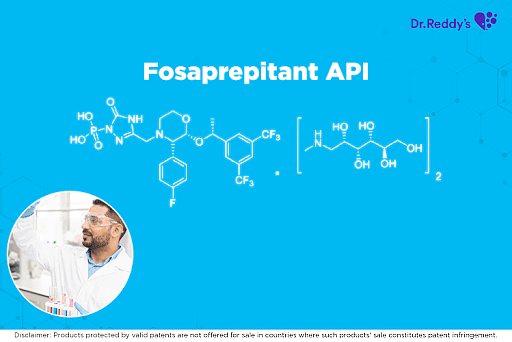
瑞迪博士的福沙吡坦(Fosaprepitant)原料药产品
Apr 15, 2021
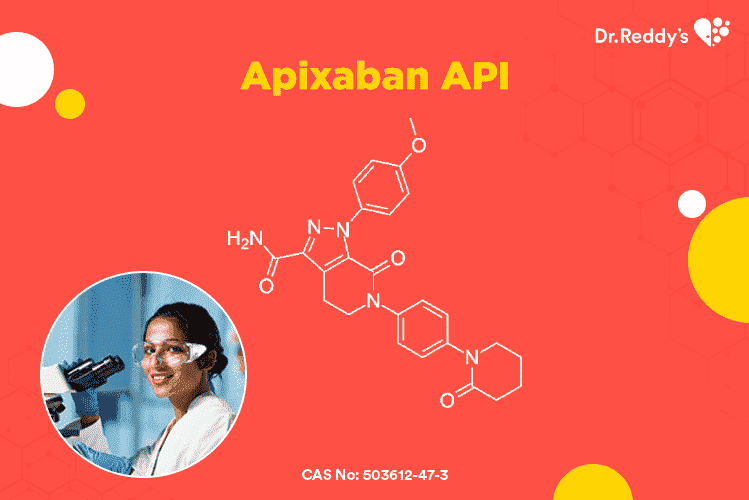
阿哌沙班(Apixaban)原料药 - 易压缩颗粒和成品剂型
Apr 6, 2021
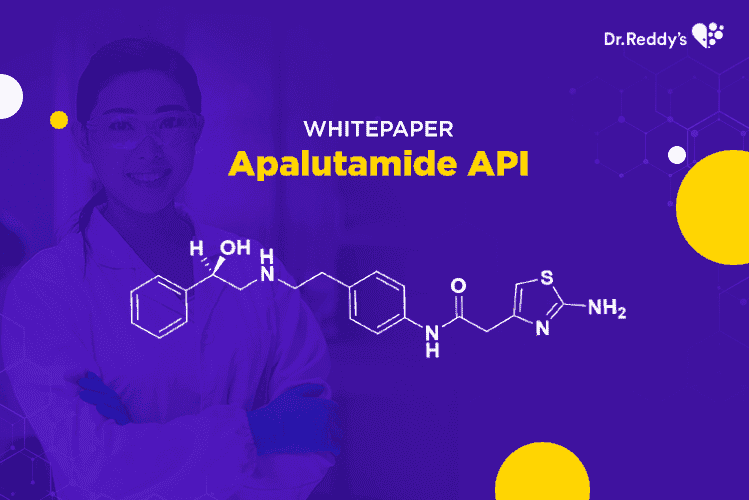
阿帕鲁胺(Apalutamide)原料药 - 对仿制药公司极具吸引力的NCE-1机遇

Apr 6, 2021
胶体铁络合物(Colloidal Iron Complex)配方 - 认识和发展流程

Feb 9, 2021
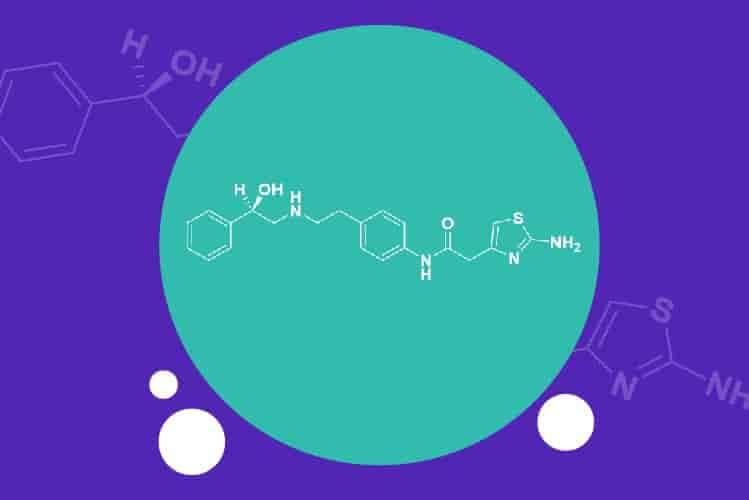
瑞迪博士的利伐沙班(Rivaroxaban): 完整的原料药和成品配方产品
Jan 22, 2021
瑞迪博士提供的阿尔法型和非晶型(Mirabegron) 原料药

Jan 13, 2021
帕博西尼(Palbociclib) - 新流程开发方法如何提供知识产权优势

Nov 25, 2020
瑞迪博士的恶拉戈利(Elagolix )原料药提供了全面的质量源于设计方法

Jul 20, 2020
沙库必曲/缬沙坦(Sacubitril/Valsartan)(LCZ696) - 通过创新的原料药产品带来另一个优势

Jul 10, 2020
瑞迪博士流动化学专家在《今日化学》(Chemistry Today)小组讨论会上分享了他们的观点

May 20, 2020
进一步了解瑞迪博士如何满足雷诺嗪(Ranolazine)中基因毒性杂质的严格监管要求

May 7, 2020
法莫替丁(Famotidine)和尼扎替丁(Nizatidine)原料药: 受控的亚硝基二甲胺(NDMA),定制的颗粒分布,安全的供应链

Oct 17, 2019
满足沙坦(Sartan)原料药生产的新法规要求
Articles

Dec 13, 2025
Spray Drying: Advancing Pharmaceutical Performance Through Precision Engineering

Nov 16, 2025
Understanding DMF‑Quality APIs and Their Key Applications in Global Pharma

Oct 12, 2025
API Process Development: Turning Molecules into Manufacturable, Scalable, High-Quality APIs

Sep 9, 2025
Zero Liquid Discharge (ZLD) in Pharma API Manufacturing

Aug 14, 2025
FDA GMP Compliance for APIs Documentation, Validation, and Quality Systems

Jul 25, 2025
Development of Niche Products at Dr. Reddys API

Jun 6, 2025
Harnessing India's New Drug Development Capabilities for Global Impact

May 6, 2025
The Impact of Global Regulations on the Active Pharmaceutical Ingredient Industry

Apr 8, 2025
The Importance of Supply Chain Management and Dr. Reddy’s Excellence

Mar 5, 2025
Current Landscape of Cancer Treatment in India and Dr. Reddy's Role in Enhancing Care

Feb 10, 2025
Global Development Strategy for Generic Formulations and APIs

Jan 6, 2025
APIs for Emerging Markets: Opportunities and Challenges

Dec 15, 2024
Formulation Strategies and Pharmaceutical Analysis for Anti-diabetic APIs

Dec 2, 2024
API Quality Control Technologies & Strategies: Current Advancements for Benefiting Pharma Companies

Oct 9, 2024
A year of growth & Sustainability
Aug 30, 2024
Understanding Nitrosamines and GTI Issues in APIs: A Comprehensive Overview with Dr. Reddy's Perspective

Aug 28, 2024
Innovative Approaches to API Production in 2024: Shaping the Future of Pharma Companies

Jul 30, 2024
Continuous Manufacturing Process and Its Impact on Pharma Manufacturing

Jun 28, 2024
A Comparative Study on Naproxen Formulations

Jun 18, 2024
An effective strategy for the development of docetaxel
May 22, 2024
Preformulation Studies for Generic Omeprazole

May 15, 2024
A comparative study on enzalutamide Formulations

Apr 30, 2024
Navigating the Heart of Pharmaceuticals: An In-Depth Exploration of Active Pharmaceutical Ingredient Manufacturing for Essential Medicines
Apr 1, 2024
Presence of Organic Impurities in active pharmaceutical ingredients: An Overview

Mar 30, 2024
Strategies and Technologies for Enhancing Cost-Effectiveness in Active Pharmaceutical Ingredient (API) Production

Mar 29, 2024
Effective Formulation Development Strategies for Poorly Soluble Active Pharmaceutical Ingredients (APIs)

Feb 29, 2024
Ensuring Drug Safety: Comprehensive Analysis of Active Pharmaceutical Ingredient Lists
Feb 28, 2024
Unravelling the Science: Pharmaceutical Compositions and Process of Levetiracetam API

Nov 16, 2023
Impact of Venetoclax Exposure on Clinical Efficacy and Safety in Patients with Refractory Chronic Lymphocytic Leukemia

Aug 10, 2023
Demonstrating Equivalence of Generic Glatiramer Acetate: Ensuring Quality and Safety
May 25, 2023
Streamlining the Process: Preparing a Pharmaceutical Composition of Fosaprepitant API
Dec 30, 2022
Steps taken by Dr. Reddy's Laboratories in controlling the environmental risks and safety management in the manufacturing of APIs
Dec 7, 2022
Meeting the New Regulatory Requirements and Controlling of Nitrosamine Impurities in Sartan API Production

Sep 14, 2022
Effective Formulation Development Strategies for Poorly Soluble Active Pharmaceutical Ingredients
Sep 13, 2022
Reshaping the Pharmaceutical API Industry with Digital Transformation

Aug 2, 2022
7 Ways how Active Pharmaceutical Ingredients (APIs) Enable Indian Pharmaceutical Companies to Grow

Aug 2, 2022
How is the Indian Pharmaceutical industry gearing up for 2022-2023?
Apr 14, 2022
Impact of COVID-19 on Indian HPAPI industry
Apr 14, 2022
The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry
Sep 14, 2021
瑞迪博士的61种原料药均采用巴西卫生监督局(ANVISA)认证的良好生产规范(GMP) 药品成分
Jul 7, 2021
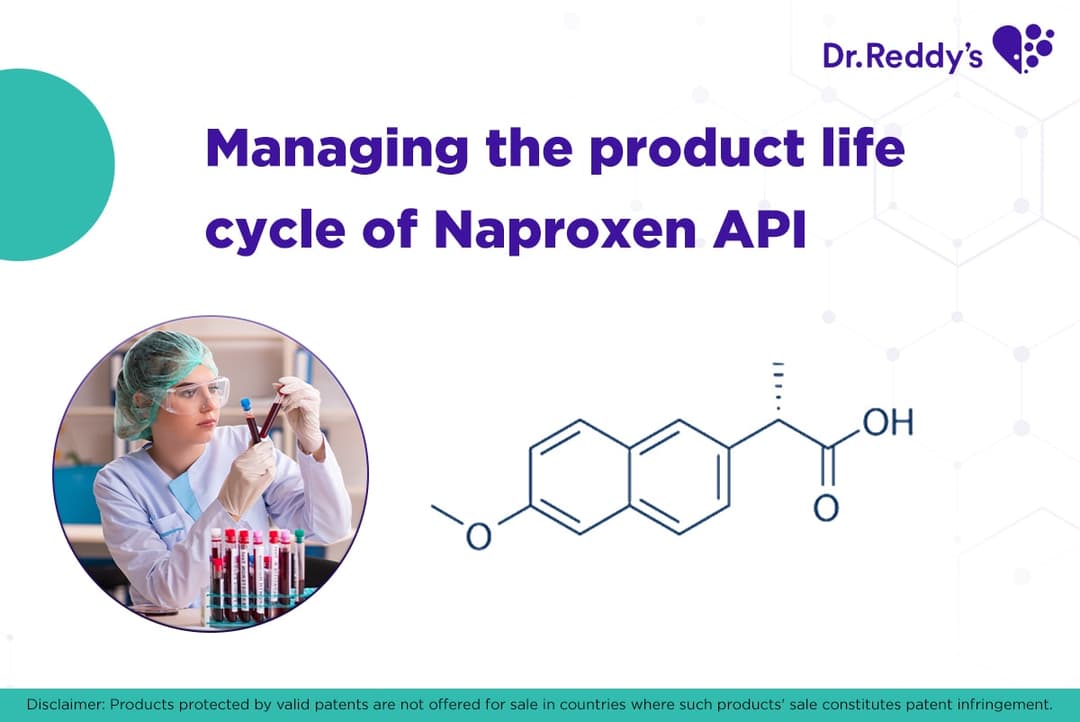
产品生命周期管理:萘普生(Naproxen)原料药和配方

May 27, 2021
了解原料药: 拉马尼·苏萨拉 (Ramani Susarla)博士的制药原料展览会(CPHI)演讲
Mar 31, 2021
EMA Nitrosamine Guidance – Deadline March 31, 2021

Nov 26, 2020
2021年患者为中心和供应链安全 - 拉杰什·萨达南丹 (Rajesh Sadanandan)就2021年世界医药原料展(CPhI)制药趋势发表了讲话

Nov 19, 2020
瑞迪博士关于在单颗粒水平上同时进行自动图像分析和拉曼光谱的研究出版物

Sep 14, 2020
范式转变将持续 - 索里•古德拉瓦莱蒂(Sauri Gudlavalleti)与CME经理谈论新冠病毒病应对措施

Sep 7, 2020
充满挑战与机遇的一年 - 制药服务和活性成分(PSAI)全球主管迪帕克•萨普拉接受了《药物厂商》(The Medicine Maker)的采访

Sep 2, 2020
新冠病毒病和原料药采购的下一步是什么?

Aug 25, 2020
印度医疗改革提供了蓬勃发展的医药市场机会: 采访迪帕克·萨普拉(Deepak Sapra)

Jun 1, 2020
瑞迪博士正在通过大规模生产能力和后向一体化来改善普瑞巴林(Pregabalin)原料药的供应链绩效

Dec 1, 2019
Enhancing Supply Chain Security for Levetiracetam API
News

Dec 15, 2025
CPHI Frankfurt 2025 - Gold Certificate for Best Stand for Sustainability

Dec 15, 2025
Dr. Reddys API Calendar Lauch 2026

Dec 12, 2025
The World of HIV: Invisible Battles, Visible Hope” symposium at the Leadership Academy in Bachupally, Hyderabad

Nov 28, 2025
Dr. Reddys API team at CPHI India 2025

Oct 28, 2025
Dr. Reddys API team at CPHI Frankfurt 2025

Oct 10, 2025
A Bold Leap for Global HIV Prevention: Lenacapavir Agreement Signed

Aug 5, 2025
Tentative Approval for Generic Lumateperone capsules

Jul 10, 2025
CDP Recognition

Jun 24, 2025
Dr. Reddys API team at CPHI China 2025

Apr 9, 2025
CPHI Japan 2025 - Exhibitor showcase session by Nirav K Shah, Vice President, Head API, North Asia

Apr 9, 2025
Dr. Reddys API team at CPHI Japan 2025

Mar 17, 2025
Dr. Reddys API team at DCAT 2025

Feb 25, 2025
Dr. Reddy’s Wins Big at “Express Pharma Excellence Awards 2025”

Dec 18, 2024
Celebrating Creativity and Festivity: API Calendar Launch 2025!

Nov 29, 2024
Dr. Reddy’s wins CPHI & PMEC India Award for Excellence in Ancillary Pharma Services - Supply Chain & Logistics for enhancing customer experience

Nov 29, 2024
Dr. Reddy’s wins CPHI & PMEC India Award for developing a continuous API manufacturing process.

Nov 29, 2024
Dr. Reddy's API team at CPHI India 2024

Oct 13, 2024
Dr. Reddy's API team at CPHI Milan 2024

Sep 27, 2024
Gunjan Singh at the CPHI Webinar: The Changing Dynamics of Global API Manufacturing

Sep 27, 2024
Karun Gaur at CPHI Milan: Panel Discussion on Pharma Manufacturing in Emerging Markets - Moving Towards Localization in Africa

Jul 8, 2024
Deepak Sapra's Visionary Keynote on Healthcare Innovation at DXEM 2024

May 8, 2024
Dr. Reddy's AI Day: Igniting the Future of Healthcare with Innovation

Apr 25, 2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

Apr 22, 2024
Dr. Reddy’s API at CPHI Japan 2024

Apr 5, 2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

Jan 2, 2024
Dr. Reddy's API Calendar 2024 Unveiling: A Magical Celebration of Creativity, Joy, and Togetherness with Our Global Team's Talented Young Artists

Dec 5, 2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

Nov 30, 2023
Dr. Reddy’s at CPHI India 2023

Nov 23, 2023
Dr. Reddy’s at 5th CII LIFE SCIENCES SUMMIT 2023 - Making India a Global Pharma and Lifesciences Innovation Hub

Nov 11, 2023
Asia-Pacific Climate Leader Award

Nov 10, 2023
Dr. Reddy’s Achieves AEO-T3 Certification, Elevating Global Trade Facilitation

Oct 28, 2023
Dr. Reddy’s API at CPHI Worldwide 2023

Aug 20, 2023
Dr. Reddys at G20 Summit 2023

Jun 25, 2023
Dr. Reddy’s API at CPHI China 2023

Mar 15, 2023
Being Prepared for What's Next and a Shared Purpose of Serving Patients Whatever it Takes

Feb 15, 2023
Dr. Reddy’s included in Bloomberg Gender-Equality Index for the 6th year in a row and S&P Global’s Sustainability Yearbook for the 3rd year

Jan 5, 2023
Dr. Reddy's API calendar 2023

Nov 11, 2022
COVID-19: Prime Minister of India Meets Pharma leaders

Nov 6, 2022
Addressing NDMAs in Pharmaceuticals

Nov 5, 2022
Dr.Reddy's at CPHI India 2022 Panel Discussion

Nov 4, 2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

Oct 6, 2022
Dr. Reddy's API participated in the 2022 Pharmaceutical Industry and Regulators Symposium at ANVISA

Sep 27, 2022
Expert statement by Srividya Ramakrishnan, Head, API process engineering, Dr. Reddy’s Laboratories. Published in CHE Manager

Sep 25, 2022
Dr. Reddys attended ACHEMA 2022 Conference

Nov 11, 2021
Dr. Reddy’s API team is a 2021 winner in – API Supplier of the year at Global generics & Biosimilars awards

Nov 11, 2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

Oct 7, 2021
2021年美国制造业最佳印度公司的获胜者

Oct 6, 2021
舒更葡糖钠原料药 - 巴西卫生监督局批准的原料药档案适当性函

May 27, 2021
迪帕克•萨普拉谈到了印度国防研究与发展组织的抗新冠病毒药物2DG

May 27, 2021
Dr.Reddy’s gearing up with Covid portfolio: Deepak Sapra, CEO of API & Services, speaks to India Today

Jan 21, 2021
2021年度制药行业最佳供应链团队

Nov 10, 2020
瑞迪博士实验室的阿尼尔•帕特尼 (Anil Patni)在《制造化学家》(Manufacturing Chemist)现场虚拟会上介绍了高效原料药(HPAPIs)

Nov 3, 2020
瑞迪博士实验室在2020年全球仿制药和生物仿制药奖(Global Generics & Biosimilars Awards)上获得“年度原料药供应商”奖

Oct 19, 2020
瑞迪博士在2020年世界医药原料展制药奖(CPhI Pharma Awards 2020)上获得了“卓越制药:可持续发展”类别的荣誉:

Oct 13, 2020
世界医药原料展制药节圆桌会议: 解决亚硝胺污染问题

Oct 5, 2020
瑞迪博士的实验室加入了科学基础目标计划(SBTi),制定了2030年的温室气体排放目标

Oct 5, 2020
世界医药原料展制药节专题讨论:跨境创新: 制药公司和创新者如何抗击新冠病毒病以及前进的道路。

Jul 1, 2020
瑞迪博士与富士胶片和Avigan®(法匹拉韦)全球响应援助机构(Global Response Aid)合作,Avigan®是一种潜在的新冠病毒病治疗药物

Apr 14, 2020
瑞迪博士关于新冠病毒病大流行的报告

Apr 1, 2020
《全球制药视野》上的一篇题为《亚硝胺污染:制药公司对其供应链的重新评估》的文章特别介绍了瑞迪博士。

Apr 1, 2020
左氧氟沙星半水合物(Levofloxacin Hemihydrate)获得欧洲药典适用性认证(CEP)

Mar 1, 2020
阿帕鲁胺的美国药物主文件 - 现已可用

Mar 1, 2020
瑞迪博士2018-19年可持续发展报告

Dec 1, 2019
瑞迪博士的原料药团队搬进其在中国的新办公室

Nov 1, 2019
瑞迪博士的实验室在2019年全球仿制药和生物仿制药奖上获得“年度原料药供应商”奖
Webinars
Updates
免责声明
本網站上的任何信息,包括對任何產品或服務的任何提及,均不構成銷售要約或被解釋為代表銷售要約。受有效專利保護的產品不得提供或供應用於商業用途。但是,在某些情況下,雷迪博士可自行決定並根據當地法律要求,在存在此類監管豁免的任何地方,提供此類產品的研究數量,以根據《印度專利法》第 107A 條(Bolar 豁免)進行監管提交。購買者應對其各自市場的產品或服務(包括專利情況)進行獨立評估,並對所有與專利相關的責任負責。 Dr. Reddy's 不承擔任何明示或暗示的保證,包括但不限於適銷性、適用於特定用途和非侵權的保證。
