Our Team at CPHI India 2024

Deepak Sapra
CEO, Pharmaceutical Services & Active Ingredients

Azhar Khan
Vice President & Head, API - Emerging Markets

Siddhartha Deshpande
Associate Vice President, API Sales & Marketing, SEAMEA

Vishal N Bhojak
Director, API Sales & Marketing - SSEA

Susmit Dey
Director, API Sales & Marketing - SSEA

Netraja Jitendra Patil
Associate Director, API Sales & Marketing - Turkey, Jordan & Africa

Ashwinikumar Kulkarni
Vice President & Global Head - Demand Planning, Customer Support & Program Management
About Dr.Reddy’s API Business
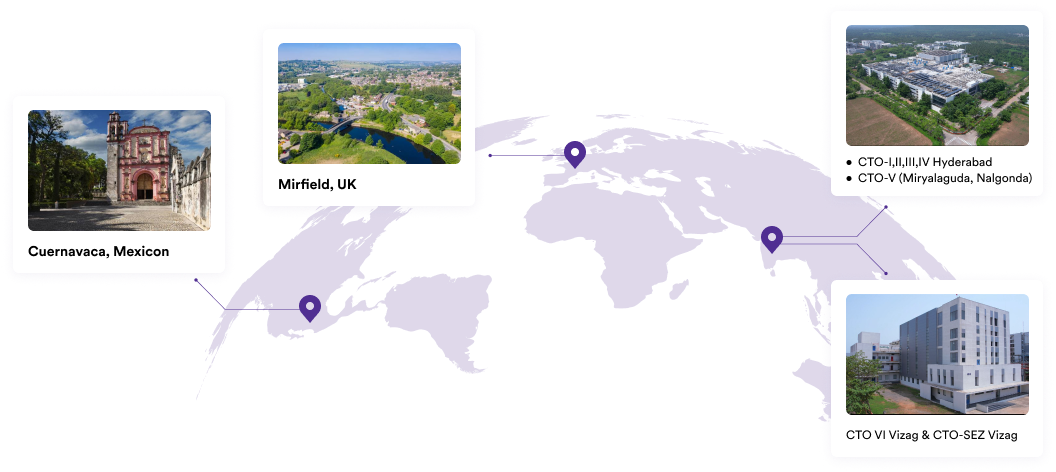
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
-
250+ APIs
-
8 Manufacturing sites
-
1900 drug master files across global markets.
-
Over 170 ANDAs and 500 DMFs
-
More than 1000 patents field, and more than 70 own patents granted
-
Experience with complex APIs and formulations supporting early market entries.
-
Preferred pharma API supplier for pharma companies in more than 80 countries.
Sustainability is deeply embedded in our purpose and forms the core of our organization.
Our journey began with APIs in 1984, and we evolved from small molecules to large molecules and from APIs to finished formulations over the decades. Amidst this evolution, what has remained constant at the heart of our organization is a deep commitment to patients and the customers we serve through our core principles of collaboration and sustainability.
By the end of FY2024, we had served over 700 million patients and continue our endeavours to reach over 1.5 billion patients globally by 2030. This means almost one in every five people on this planet will use Dr. Reddy’s product or service. The health of people is strongly linked to the health of our planet. That's why our integrated report also shows how sustainability and business are strongly linked.
What we achieved in the past year:


Our Achievement (awards)

25/04/2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

05/04/2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

05/12/2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

04/11/2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

11/11/2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

19/10/2020
Dr. Reddy's recognized at the CPhI Pharma Awards 2020 as the winner in the category of ‘Excellence in Pharma: Sustainability’
Our Plants
About CPHI India 2024
CPHI India is the leading pharmaceutical event in the region, uniting suppliers, buyers, and industry professionals across the entire pharmaceutical supply chain. This annual gathering takes place in New Delhi, offering an unparalleled platform for the Indian pharma community to connect, collaborate, and innovate.

Key Highlights of CPHI India 2024:
-
Extensive Networking Opportunities: Engage with thousands of pharma professionals from India and around the world, fostering connections that can lead to fruitful collaborations.
-
Diverse Exhibitor Showcase: Explore a comprehensive list of exhibitors spanning every sector of the pharmaceutical market, providing insights into the latest products and services.
-
Educational Insights: Participate in conferences and discussions led by industry leaders, gaining valuable knowledge and perspectives on emerging trends and challenges.
-
Cost-Effective Solutions: Discover innovative advancements and solutions tailored to optimize your business operations and enhance efficiency.
CPHI India 2024 serves as the perfect venue to expand your business network, uncover new technologies, and forge reliable partnerships. Join us in shaping the future of the pharmaceutical industry.
Renuncia
Ninguna información en este sitio web, incluyendo cualquier referencia a cualquier producto o servicio, constituye una oferta de venta ni se interpretará como tal. Los productos protegidos por patentes válidas no se ofrecen ni suministran para uso comercial. Sin embargo, en ciertos casos, a discreción exclusiva de Dr. Reddy's y sujeto a los requisitos legales locales, las cantidades de investigación de dichos productos pueden ofrecerse para fines de presentaciones regulatorias según la Sección 107A de la Ley de Patentes de la India (exención de Bolar), donde existan dichas exenciones regulatorias. Los compradores deben realizar su propia evaluación del producto o servicio, incluyendo el escenario de patentes en sus respectivos mercados, y serán responsables de todas las responsabilidades relacionadas con las patentes. Dr. Reddy's renuncia a todas las garantías, expresas o implícitas, incluyendo, entre otras, las garantías de comerciabilidad, idoneidad para un propósito particular y no infracción.
Let’s discuss API sourcing

Join us at Hall - 9.1 | Booth - B48






