Our Team at CPHI India 2024

Deepak Sapra
CEO, Pharmaceutical Services & Active Ingredients

Azhar Khan
Vice President & Head, API - Emerging Markets

Siddhartha Deshpande
Associate Vice President, API Sales & Marketing, SEAMEA

Vishal N Bhojak
Director, API Sales & Marketing - SSEA

Susmit Dey
Director, API Sales & Marketing - SSEA

Netraja Jitendra Patil
Associate Director, API Sales & Marketing - Turkey, Jordan & Africa

Ashwinikumar Kulkarni
Vice President & Global Head - Demand Planning, Customer Support & Program Management
About Dr.Reddy’s API Business
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
-
250+ APIs
-
8 Manufacturing sites
-
1900 drug master files across global markets.
-
Over 170 ANDAs and 500 DMFs
-
More than 1000 patents field, and more than 70 own patents granted
-
Experience with complex APIs and formulations supporting early market entries.
-
Preferred pharma API supplier for pharma companies in more than 80 countries.
Sustainability is deeply embedded in our purpose and forms the core of our organization.
Our journey began with APIs in 1984, and we evolved from small molecules to large molecules and from APIs to finished formulations over the decades. Amidst this evolution, what has remained constant at the heart of our organization is a deep commitment to patients and the customers we serve through our core principles of collaboration and sustainability.
By the end of FY2024, we had served over 700 million patients and continue our endeavours to reach over 1.5 billion patients globally by 2030. This means almost one in every five people on this planet will use Dr. Reddy’s product or service. The health of people is strongly linked to the health of our planet. That's why our integrated report also shows how sustainability and business are strongly linked.
What we achieved in the past year:


Our Achievement (awards)

25/04/2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

05/04/2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

05/12/2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

04/11/2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

11/11/2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

19/10/2020
Dr. Reddy's recognized at the CPhI Pharma Awards 2020 as the winner in the category of ‘Excellence in Pharma: Sustainability’
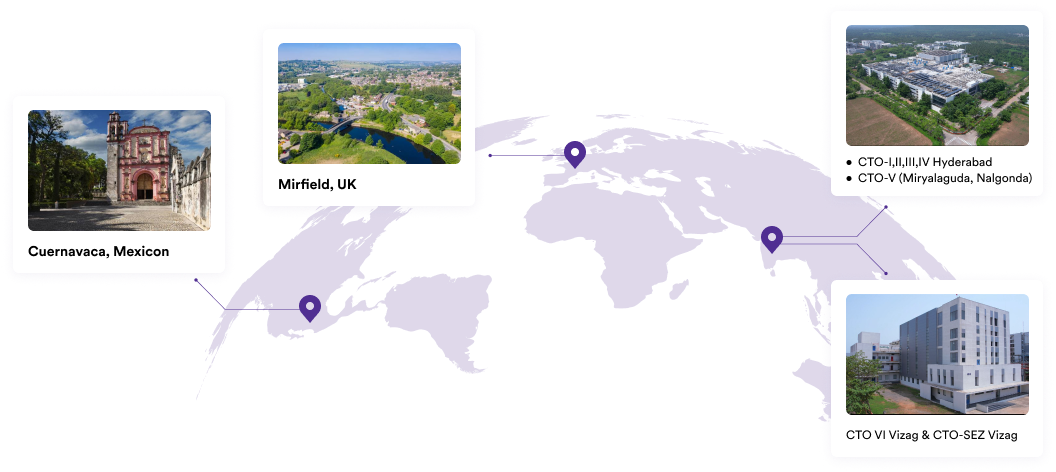
Our Plants
About CPHI India 2024
CPHI India is the leading pharmaceutical event in the region, uniting suppliers, buyers, and industry professionals across the entire pharmaceutical supply chain. This annual gathering takes place in New Delhi, offering an unparalleled platform for the Indian pharma community to connect, collaborate, and innovate.

Key Highlights of CPHI India 2024:
-
Extensive Networking Opportunities: Engage with thousands of pharma professionals from India and around the world, fostering connections that can lead to fruitful collaborations.
-
Diverse Exhibitor Showcase: Explore a comprehensive list of exhibitors spanning every sector of the pharmaceutical market, providing insights into the latest products and services.
-
Educational Insights: Participate in conferences and discussions led by industry leaders, gaining valuable knowledge and perspectives on emerging trends and challenges.
-
Cost-Effective Solutions: Discover innovative advancements and solutions tailored to optimize your business operations and enhance efficiency.
CPHI India 2024 serves as the perfect venue to expand your business network, uncover new technologies, and forge reliable partnerships. Join us in shaping the future of the pharmaceutical industry.
免責事項
このウェブサイト上のいかなる情報も、製品またはサービスへの言及を含め、販売の申し出を構成するものではなく、販売の申し出を表すものと解釈されるものでもありません。有効な特許により保護されている製品は、商用目的で提供または提供されるものではありません。ただし、特定のケースでは、Dr. Reddy の独自の裁量により、現地の法的要件に従って、そのような製品の研究用数量が、規制免除が存在する場所に、インド特許法第 107A 条 (Bolar 免除) に基づく規制提出の目的で提供される場合があります。購入者は、それぞれの市場における特許シナリオを含む製品またはサービスについて独自の評価を行う必要があり、すべての特許関連法的責任を負うことになります。Dr. Reddy は、商品性、特定目的への適合性、および非侵害の保証を含むがこれらに限定されない、明示または黙示を問わずすべての保証を否認します。
Let’s discuss API sourcing

Join us at Hall - 9.1 | Booth - B48






