Our Team at CPHI Milan 2024

Deepak Sapra
CEO, Pharmaceutical Services & Active Ingredients

Gunjan Singh
Vice President and Head, API - Mature Markets

Azhar Khan
Vice President and Head, API - Emerging Markets

Dr. NM Sekhar
Dr. NM Sekhar, Vice President, API R&D

B Vivek
Executive VP & Global Head - Supply, Demand Planning & Delivery

Dr. A Kalyan Chakravarthy
Executive VP & Head – SEAMEA & B2B Formulations

Gustavo Mayrink De Castro Marques
Vice President and Head, API Sales & Marketing, LATAM

Kalpesh Modi
Vice President, and Head – EU/CIS Market, API & B2B Collaborations

Karun Gaur
Vice President and Head, Strategic Projects API

R Lalitha Kumari
Global Head of API Product Management & Head of Sales - US & Canada

Nirav Shah
Vice President, and Head, API - North Asia

Rohan Kaushik
Associate Vice President, API - BD & Portfolio

Siddhartha Deshpande
Associate Vice President, API Sales & Marketing, SEAMEA

Pratik Singh Bais
Director, API - Sales & BD, Mexico & Canada

Rohit Jayant Dash
Rohit Jayant Dash, Director - API Sales & Marketing, GCC, Africa & Turkey

Ashish Rana
Ashish Rana, Director, API - BD & Sales, Europe

Yuri Alberto Pereira
Director, API Sales & Marketing – LATAM
About Dr.Reddy’s API Business
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 150+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.
-
250+ APIs
-
8 Manufacturing sites
-
More than 1873 drug master files across global markets.
-
Over 325 ANDAs
-
More than 1000 patents field, and more than 70 own patents granted
-
Experience with complex APIs and formulations supporting early market entries.
-
Preferred pharma API supplier for pharma companies in more than 80 countries.

Sustainability is deeply embedded in our purpose and forms the core of our organization.
Our journey began with APIs in 1984, and we evolved from small molecules to large molecules and from APIs to finished formulations over the decades. Amidst this evolution, what has remained constant at the heart of our organization is a deep commitment to patients and the customers we serve through our core principles of collaboration and sustainability.
By the end of FY2024, we had served over 700 million patients and continue our endeavours to reach over 1.5 billion patients globally by 2030. This means almost one in every five people on this planet will use Dr. Reddy’s product or service. The health of people is strongly linked to the health of our planet. That's why our integrated report also shows how sustainability and business are strongly linked.
What we achieved in the past year:


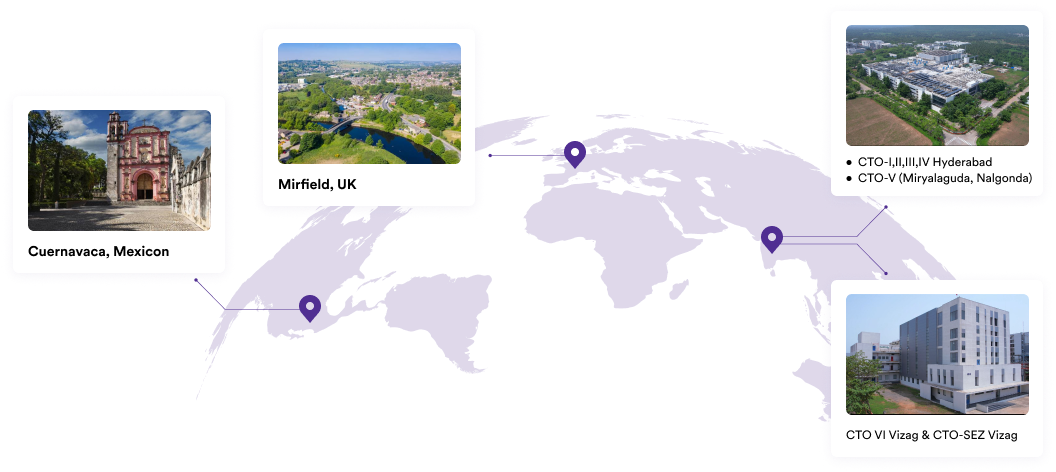
Our Plants
About CPHI Milan 2024
CPHI Milan is a global pharmaceutical event uniting the pharmaceutical community to advance human health. With a history spanning 35 years, the event has become a cornerstone for fostering connections and inspiring partnerships across the industry. CPHI Milan is a platform for professionals to engage with the latest innovations, share knowledge, and collaborate on projects that shape the future of pharmaceuticals.

Key Highlights of CPHI Milan 2024:
-
Global Participation: The event expects to welcome 62,000 attendees and 2,400 exhibitors from 168+ countries, demonstrating its significant international reach.
-
Diverse Exhibition: Attendees can explore a wide range of products and services across various pharmaceutical sectors.
-
Networking Opportunities: CPHI Milan offers numerous opportunities for attendees to connect, build relationships, and forge partnerships.
-
Knowledge Exchange: The event features conferences, seminars, and workshops where industry experts share insights and discuss the latest trends.
-
Celebration of 35 Years: CPHI Milan 2024 marks a special milestone, celebrating 35 years of uniting the pharma community.
Our Achievement (awards)

25/04/2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

05/04/2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

05/12/2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

04/11/2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

03/11/2021
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2021

11/11/2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

19/10/2020
Dr. Reddy's recognized at the CPhI Pharma Awards 2020 as the winner in the category of ‘Excellence in Pharma: Sustainability’
免责声明
本網站上的任何信息,包括對任何產品或服務的任何提及,均不構成銷售要約或被解釋為代表銷售要約。受有效專利保護的產品不得提供或供應用於商業用途。但是,在某些情況下,雷迪博士可自行決定並根據當地法律要求,在存在此類監管豁免的任何地方,提供此類產品的研究數量,以根據《印度專利法》第 107A 條(Bolar 豁免)進行監管提交。購買者應對其各自市場的產品或服務(包括專利情況)進行獨立評估,並對所有與專利相關的責任負責。 Dr. Reddy's 不承擔任何明示或暗示的保證,包括但不限於適銷性、適用於特定用途和非侵權的保證。
Let’s discuss API sourcing

Join us at Hall - 9.1 | Booth - B48






