Our Team at DCAT Week 2026
About Dr.Reddy's API Business
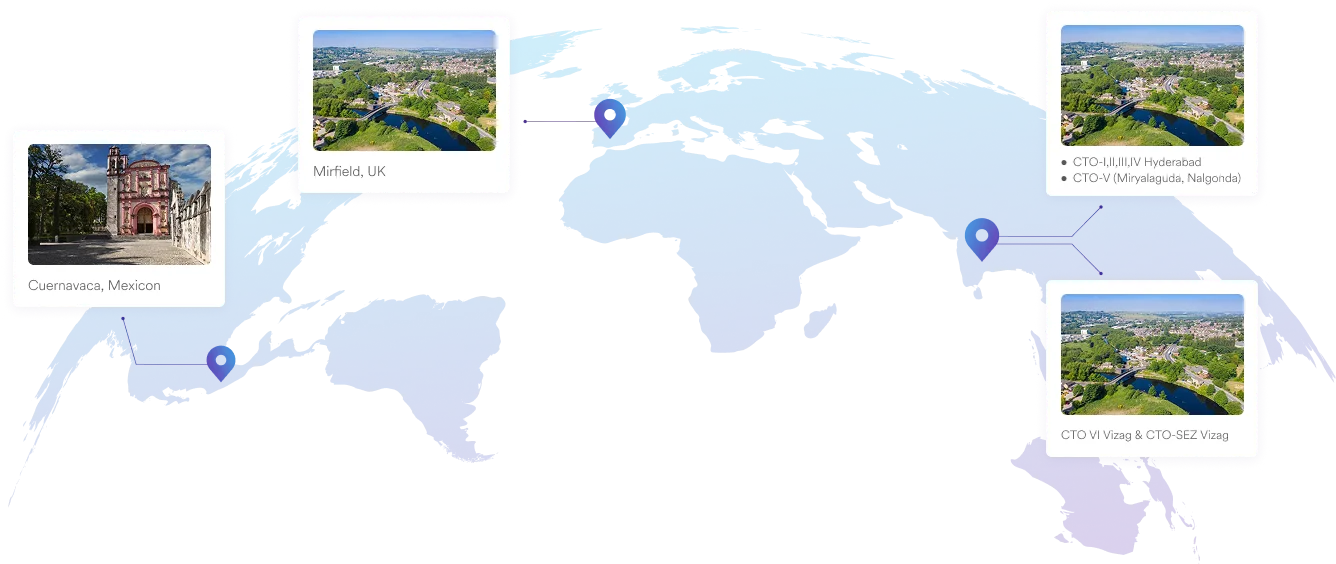
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 250+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy’s API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.

250+ APIs

8 Manufacturing sites

1970+ drug master files across global markets.

Over 325 ANDAs

Filed4500+ patents globally, out of which 1125 have been granted.

Experience with complex APIs and formulations supporting early market entries

Preferred pharma API supplier for pharma companies in more than 80 countries

Sustainability is deeply embedded in our purpose and forms the core of our organization.
Our journey began with APIs in 1984, and we evolved from small molecules to large molecules and from APIs to finished formulations over the decades. Amidst this evolution, what has remained constant at the heart of our organization is a deep commitment to patients and the customers we serve through our core principles of collaboration and sustainability. By the end of FY2025, we had served over 756 million patients and continue our endeavours to reach over 1.5 billion patients globally by 2030. This means almost one in every five people on this planet will use Dr. Reddy’s product or service. The health of people is strongly linked to the health of our planet. That's why our integrated report also shows how sustainability and business are strongly linked.
Our ESG (Environment, Social & Governance) Goal:
Building the future responsibly
Our ESG goals are deeply embedded in our business strategy, guiding our efforts to create a significant and lasting positive impact on the planet and its people. We have made fair progress across several of these goals, even as a few continue to present challenges. We are committed to advancing our ESG journey, ensuring that as we prosper, we do so responsibly and with resilience.
What we achieved in the past year:
3.8
Bn total revenue (17% growth)
165
New products launched
111
DMFs filed, (1,630+ Cumulative DMFs)
249
Dossiers, including 10 ANDAs filed
76
Filings in the US pending for approval
20.4%
Women in leadership
96%
Employees trained for skill upgradation
0.14 to 0.07
*LTIFR reduced from
126
Differently abled employees
700,000+
Lives impacted through CSR
783
Million KL of water saved through agri-water saving techniques
66,723
MTCO2e of direct emissions reduction
11%
Reduction in water intensity per rupee of turnover with respect to FY2024
60%
Carbon neutrality achieved
75,761
KL of harvested rainwater used
54%
Water reused/recycled
Our Plants

Dr. Reddy’s Participation at DCAT Week 2026
DCAT Week 2026 is one of the most prominent annual gatherings for the global pharmaceutical and chemical manufacturing community. For Dr. Reddy’s Laboratories, it serves as a strategic platform to strengthen engagement with global partners, highlight capabilities, and reinforce its leadership in the API (Active Pharmaceutical Ingredients) space.
At DCAT, our API business plays a central role in showcasing Dr. Reddy’s end‑to‑end strengths—from a broad product portfolio and high-quality manufacturing to our commitment to sustainability and innovation across the value chain. The event enables our teams to conduct focused meetings with customers, suppliers, and industry collaborators to drive long‑term business growth.
What Dr. Reddy’s Does at DCAT Week 2026
Showcases a Robust API Portfolio
We present our extensive offering of 250+ high‑quality APIs across key therapeutic areas including oncology, cardiovascular, gastrointestinal, central nervous system, and anti-infectives. This demonstrates our capability to support diverse customer requirements globally.
Engages with Global Stakeholders
DCAT provides a forum for our commercial, sourcing, and leadership teams to interact with existing partners and explore new collaborations. These discussions often translate into strategic supply agreements and long-term business partnerships.
Drives Opportunities for Growth & Collaboration
Our teams use DCAT to identify emerging market needs, exchange insights on industry trends, and explore avenues for co-development, technology partnerships, and portfolio expansion.
Highlights Our Sustainability Vision
We take the opportunity to communicate our strong ESG performance, responsible manufacturing practices, and initiatives aimed at reducing environmental impact—reinforcing our commitment to a sustainable and resilient pharmaceutical supply chain.
Key Takeaways
- DCAT Week is a high-impact platform for networking, strategic discussions, and business development within the pharmaceutical and chemical supply ecosystem.
- Dr. Reddy’s API division leverages the event to strengthen relationships, showcase technical expertise, and drive growth in global API markets.
- Our participation reflects a clear commitment to quality, innovation, operational excellence, and meaningful collaborations that support customers across the world.