Addressing the presence of mutagenic Azido impurities in Sartan APIs
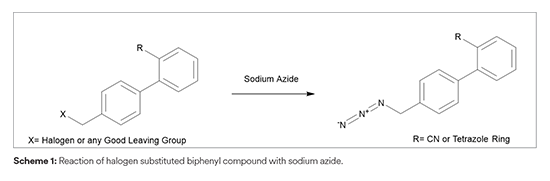
In continuation to our efforts to understand the Azido impurities, we are evaluating the possibility of formation of two additional Azido impurities - Chloro methyl azido impurity and 4-Chloro azido methyl tetrazole impurity in Losartan potassium. Based on detailed synthetic landscape, it is found that these impurities are not potentially formed in our manufacturing process. The detailed assessment report for the same would be available in the coming few weeks.
Dr. Reddy’s has continued to manufacture and deliver Sartan APIs in accordance with global regulations as a result of the processes and the analytical methods developed by our experts to avoid the presence of Nitrosamine and Azido impurities. We stay committed to ensure that we continue to provide high quality APIs to our partners.
I will be happy to connect with you for any queries or further details on our assessment on Azido impurities in Sartan APIs. You can also log onto XCEED, our customer engagement platform, to raise any sample or technical queries on the topic.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Preencha o formulário de contato abaixo para visualizar o white paper


