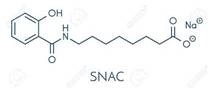
Salcaprozate Sodium (SNAC) (Excipient For Oral Semaglutide) API
CAS Number: 203787-91-1
About Salcaprozate Sodium (SNAC) (Excipient For Oral Semaglutide) API
Therapeutic Category
Others
API Technology
Synthetic
Dose Form
Oral Solids
Dr Reddy's Development Status
Available
Mechanism of Action
Salcaprozpate Sodium (SNAC) is an excipient and used as absorption enhancer for delivery of oral SemaglutideDr. Reddy's Expertise
Headquartered in Hyderabad, India, Dr. Reddy's Laboratories is one of the leading Active Pharmaceutical Ingredients (API) manufacturers and suppliers globally for Salcaprozate Sodium (SNAC) (Excipient For Oral Semaglutide) API. Dr. Reddy's API business is a preferred partner to pharma companies across the US, Europe, Brazil, Latin America, Japan, China, Korea, Middle East and other emerging markets.
Dr. Reddy's API business thrives on the deep technical strengths established over the last 30+ years in the development and manufacture of complex APIs such as steroids, peptides, complex long chain molecules and highly potent APIs (HPAPIs / oncology drugs). This expertise is complemented by our prowess in intellectual property and regulatory affairs which helps us consistently meet and exceed regulatory standards. Dr. Reddy's Salcaprozate Sodium (SNAC) (Excipient For Oral Semaglutide) API is the outcome of the extensive expertise in R&D, IP, and Regulatory.
A key component in helping our customers be first to market is a responsive supply chain. We achieve this by making sure that all our facilities are operating efficiently and to the latest standards of quality, safety, and productivity. A strong interconnect between business and factories allows for a quick reaction to dynamic market changes, so that we can avert shortages and meet sudden surges in demand.
Disclaimer
No information in this catalog - including any reference to any product or service - constitutes an offer for sale, or be construed as representing an offer for sale. Products protected under valid patents are not offered or supplied for commercial use. However, the research quantities of such products may be offered for the purpose of regulatory submissions, wherever such regulatory exemptions exist. The buyers should make their independent evaluation of the patent scenario for their respective markets and will be responsible for all patent related liabilities. Products protected under valid patents in India are not available for commercial use but would be available for Section 107A purposes.
FAQs
Enhances oral absorption of poorly bioavailable drugs like semaglutide
Available as oral tablets, typically combined with active drugs
Store at room temperature (20°C to 25°C), in a tightly sealed container, away from moisture and light
Insights Delivered
Sign-up for our email service to get Market and Product insights and updates right to your digital doorstep
The categories of personal information collected in this form include name, company, and contact information etc. The personal information collected will be used for exploratory discussions on contract manufacturing, marketing and to perform research and analytics and others. For more information about the categories of personal information collected by Dr.Reddy's and the purposes for which Dr.Reddy's uses personal information, visit https://api.drreddys.com/privacy-policy.
Disclaimer
No information on this website, including any reference to any product or service constitutes an offer for sale or be construed as representing an offer for sale. Products protected under valid patents are not offered or supplied for commercial use. However, in certain cases, at Dr. Reddy's sole discretion, and subject to local legal requirement, the research quantities of such products may be offered for the purpose of regulatory submissions under Section 107A of the Indian Patent Act (Bolar exemption), wherever such regulatory exemptions exist. The buyers should make their independent evaluation of the product or service including, patent scenario in their respective markets and will be responsible for all patent related liabilities Dr. Reddy's disclaims all warranties, express or implied, including but not limited to warranties of merchantability, fitness for a particular purpose and non-infringement.



