Regulatory Affairs: Global Expertise, Proven Compliance
Dr. Reddy’s offers unmatched regulatory expertise across international markets, ensuring our APIs meet the most stringent global standards. Our Regulatory Affairs (RA) team, with over 50 specialists, actively monitors evolving guidelines and proactively manages risk to support smooth approvals for complex APIs.
Global Reach & Proven Track Record
- DMFs filed across the U.S., Europe, Russia, Canada, China, Japan, Brazil, and more
- Expertise in DMF, CMC, IND, and NDA filings
- Strong partnerships with specialized law firms across geographies
- Facilities regularly audited by USFDA, EMA, WHO GMP, PMDA, KFDA, ANVISA, Health Canada, and others
Our regulatory strength ensures faster market access and reliable compliance for our global partners.

Experts Dedicated Team
State-of-the-art Manufacturing facilities
Total DMF(Drug Master Files) across global markets
Active DMFs
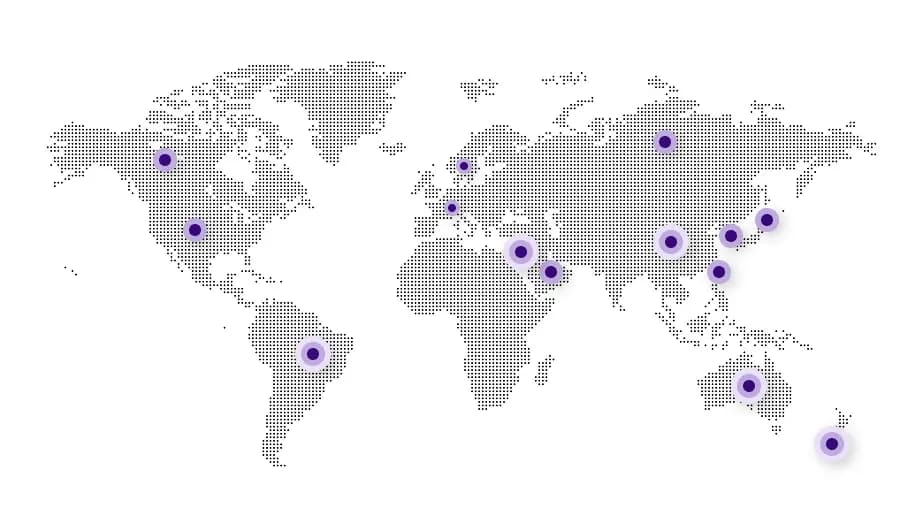
Our Global Network
- USA356
- Canada121
- Europe (ASMF)184
- Europe (CEP Filed)127
- Australia100
- New Zealand63
- Brazil174
- China115
- Israel35
- Japan57
- Korea115
- Russia56
- Saudi Arabia84
- Taiwan51
- RoW421
* As of Feb 2026
We understand the local market with broad market research & delivers high-quality regional specific DMFs to meet the regulatory requirements. Dr. Reddy's API business thrives on the deep technical strengths set up over the last 40+ years in consistently delivering high-quality APIs and manufacturing complex APIs. We follow best practices to improve our DMFs to match with the latest FDA regulatory standards. In addition, our expert Regulatory affairs & Quality teams follow the highest global standards of quality and regulatory compliance.
Regulatory Filing Updates
View AllDisclaimer
No information on this website, including any reference to any product or service constitutes an offer for sale or be construed as representing an offer for sale. Products protected under valid patents are not offered or supplied for commercial use. However, in certain cases, at Dr. Reddy's sole discretion, and subject to local legal requirement, the research quantities of such products may be offered for the purpose of regulatory submissions under Section 107A of the Indian Patent Act (Bolar exemption), wherever such regulatory exemptions exist. The buyers should make their independent evaluation of the product or service including, patent scenario in their respective markets and will be responsible for all patent related liabilities Dr. Reddy's disclaims all warranties, express or implied, including but not limited to warranties of merchantability, fitness for a particular purpose and non-infringement.

