Co-Crystal and Customized Particle Size for Early Launch Opportunity of Siponimod API
Siponimod is an oral, second-generation sphingosine-1-phosphate (S1P) receptor modulator. S1P receptor modulators can inhibit the egress and recirculation of lymphocytes from lymph nodes, a therapeutic strategy for treating certain autoimmune diseases.
Siponimod is the first and only treatment for patients with active secondary progressive multiple sclerosis (SPMS). The drug is expected to address the critical unmet need of multiple sclerosis patients in various disease categories [1].
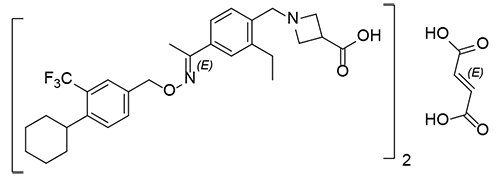
Dr. Reddy’s Siponimod API is another example of the technical capabilities of our product development team. We can provide our partners with a Siponimod Hemifumarate API, which is thoroughly characterized by its structure using techniques like MicroED, single crystal PXRD, DSC, Raman, FT-IR, and NMR (solid-state and solution). Siponimod Hemifumarate was confirmed as a co- crystal, and it exists as a 2:1 co-crystal of Siponimod and fumaric acid rather than fumarate salt.
To know more about our API offerings, please read the White Paper on Siponimod by filling the contact from below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper


