New Product Alert – Lumateperone Tosylate API
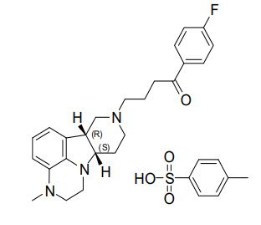
Lumateperone is a serotonin 5HT2A receptor antagonist, a dopamine receptor phosphoprotein modulator (DPPM), and a serotonin transporter (SERT) inhibitor. Unlike existing schizophrenia treatments, Lumateperone is a first-in-class molecule that provides selective and simultaneous modulation of serotonin, dopamine, and glutamate—three neurotransmitter pathways implicated in severe mental illness.
Market Overview:
The worldwide prevalence of schizophrenia is approximately 0.5 to 1%. It is one of the leading causes of disability worldwide, with a life expectancy of around 15 years less than individuals without schizophrenia.
Approximately 50% of individuals with schizophrenia experience a relapse/exacerbation in psychotic symptoms within one year after their last episode; most relapses occur in medication non-adherence.
According to global data, the bipolar disorder market is expected to grow from $4.1 billion in 2020 to $4.9 billion by 2030 at a compound annual growth rate (CAGR) of 1.7%. Furthermore, as the number of cases remains relatively stable over the forecast period (10 years), Lumateperone Tosylate is expected to become a top-three anti-psychotic drug and hit $1.2 billion in global sales across various indications by the financial year 2030-31.
To know more about our API offerings, please read the new product alert on Lumateperone Tosylate by filling the contact from below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper


