Product Alert – Mavacamten API
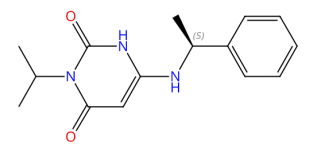
CAS no. 1642288-47-8
Mavacamten (Camzyos®) is the pioneering and singularly authorised cardiac myosin inhibitor designed to address hypertrophic cardiomyopathy (HCM) at its root cause. This unique compound acts as an allosteric and reversible inhibitor with a specific focus on cardiac myosin, allowing for the controlled adjustment of the myosin heads' activity, thus decreasing the formation of myosin-actin cross-bridges.
Dr. Reddy's API Offering
- We are developing Innovator Crystalline Form A - Labs samples; COA is available.
- Alternate polymorph/ novel form will provide 4 to 6 years of advantage.
Our cGMP API manufacturing facility has undergone a thorough inspection by international regulatory authorities, and we have reliable suppliers for key starting materials to ensure timely deliveries and adherence to strict specifications. Moreover, we are equipped to provide sufficient capacity to supply development quantities within a short lead time of 90-120 days.
To learn more about our API offerings, please read the product alert on Mavacamten API by filling out the contact form below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper

