Product Alert – Mirogabalin Besylate
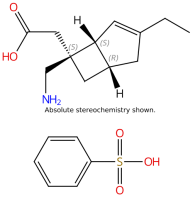
Dr. Reddy's Offerings*:
- Planning USDMF by January 2025
- Offers crystalline Form-1
- Non-GMP API and COA are available.
Quality Assurance through cGMP API Manufacturing
- Manufacturing facilities have successfully undergone inspections by international regulatory authorities.
- Partnerships with reliable key starting materials (KSM) suppliers for timely deliveries and strict adherence to specifications.
To learn more, download the product alert on Mirogabalin API HERE.
Should you have any specific queries? I would be happy to schedule a meeting at your earliest convenience.
*Note: Products under patent(s) are offered only for R&D purposes U/S 107A of the Patent Act and not for commercial sale.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper

