Tech Sheet: Dr. Reddy’s Abiraterone Acetate API offerings
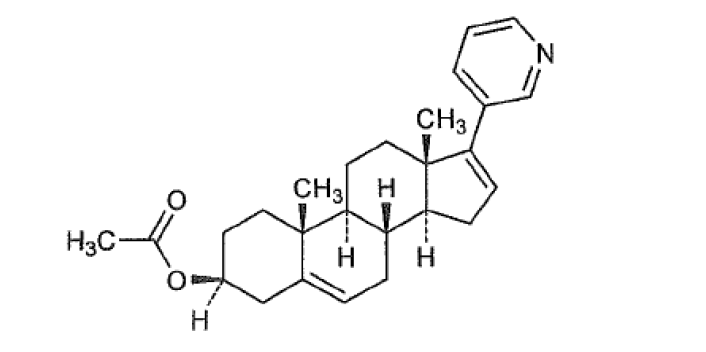
Abiraterone is an orally active inhibitor of the steroidal enzyme CYP17A1 (17 alpha-hydroxylase/C17,20 lyase). It inhibits CYP17A1 selectively and irreversibly via a covalent binding mechanism. Abiraterone is a CYP17 inhibitor indicated in combination with prednisolone to treat patients with metastatic castration-resistant prostate cancer (CRPC) and Metastatic high-risk castration-sensitive prostate cancer (CSPC).
Abiraterone Acetate (Zytiga, Janssen Biotech Inc.) was approved by the Food and Drug Administration (FDA) on April 28, 2011, for Prostate Cancer treatment. In December 2012, FDA expanded Zytiga’s use for late-stage Prostate Cancer. In February 2018, the FDA approved Abiraterone Acetate tablets with prednisone for metastatic high-risk castration sensitive prostate cancer (CSPC).
As of February 2008, Abiraterone is approved in various countries worldwide for different indications.
Key takeaways of Dr. Reddy’s Abiraterone Acetate API:
- Enough capacity to cater to current market requirements.
- Quality – in line with ICH guidelines.
- Earliest generic API manufacturer of abiraterone globally.
- Country-specific regulatory filings
- GTI & Nitrosamine free.
To know more about our offerings , please read the technical sheet on Abiraterone Acetate by filling the contact form below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper


