Tech Sheet on Midostaurin API
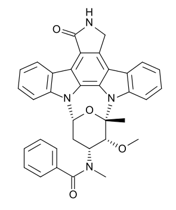
Midostaurin is a small molecule that inhibits multiple receptor tyrosine kinases. In vitro biochemical or cellular assays have shown that Midostaurin or its major human active metabolites CGP62221 and CGP52421 inhibit the activity of wild type FLT3, FLT3 mutant kinases (ITD and TKD), KIT (wild type and D816V mutant), PDGFRα/β, as well as members of the serine/threonine kinase PKC (protein kinase C) family.
Midostaurin has global sales of 188 million USD with a 74% year-over-year (YoY) growth and 58 Kg growth at 67% YoY. It is expected to become around 200 Kg market by 2025. The global Midostaurin market is projected to grow at a CAGR of 4.6% between 2022 and 2030.
The growth is primarily driven by Europe, China, and the rest of the world (ROW) markets. In addition, more approvals for novel therapies and ongoing trials for all form of Acute myeloid leukemia (AML) would further propel the market during the forecast period (2021-2030).
Dr. Reddy's API Offering
- Crystalline form II (same as innovator form) and amorphous form.
- Dr. Reddy's is among the earliest generic API manufacturers globally to file the US DMF for Midostaurin API for both polymorph forms.
- Quality by design (QbD) based API development for a consistent quality profile.
To know more about our API offerings, please read the Tech sheet on Midostaurin API by filling the contact form below.
Explore other Whitepapers:
Know More- Email us: [email protected]
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper


