Tech Sheet – Mirabegron API
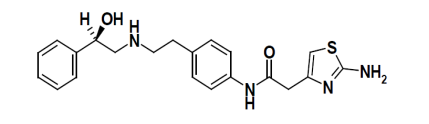
CAS No: 223673-61-8
Mirabegron is a ß-3 adrenergic receptor agonist and a widely prescribed oral drug for treating overactive bladder (OAB). The drug improves symptoms associated with OAB, such as urinary urgency or urgency incontinence.
Mirabegron was approved under the trade names Myrbetriq (US, 2012), Betmiga (Europe, 2012), and Betanis (Japan, 2011). As of July 2023, Mirabegron is approved in 60 countries worldwide for OAB indications.
Dr. Reddy's API Offering*:
- We manufacture Mirabegron at our cGMP API manufacturing facility (CTO-6, Vizag), which is successfully inspected by international regulatory authorities such as the USFDA, EMEA, ANVISA, PMDA and Health Canada.
- We offer Alpha Form-1 (same as the originator).
- API quality complies with most Stringent European Pharmacopoeia 11.2 monograph specifications.
- Employing a Quality by Design approach for efficient processes and high-quality output.
- Developing an atom-efficient process to minimise process waste.
- Effective control strategy for avoiding potential genotoxic and carcinogenic impurities.
- Assured process safety and scalability.
To learn more about our API offerings, please read the tech sheet on Mirabegron API by filling out the contact form below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper

