Tech Sheet On Nilotinib Hydrochloride
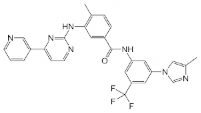
Nilotinib is an inhibitor of the BCR-ABL kinase. Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL protein. In vitro, Nilotinib inhibited BCR-ABL-mediated proliferation of murine leukemic cell lines and human cell lines derived from patients with Ph+ chronic myelogenous Leukemia (CML).
Dr. Reddy's is among the earliest generic API manufacturers globally to file the USDMF for Nilotinib HCL API (filed on July 08, 2022), and we are planning to file the EUDMF by March 2022 and we offer monohydrate from-B (Innovator form).
Our manufacturing process has been designed to address customized particle size distribution (PSD) requirements through size reduction and crystallization techniques to consistently meet the most desired PSDs on a commercial scale. With an adequate capacity to meet global demand, we manufacture Nilotinib HCL at our cGMP API manufacturing facility, successfully inspected by international regulatory authorities. In addition, we have de-risked our supply chain as the key starting materials (KSM) of our Nilotinib Hydrochloride API are backward integrated today.
To know more about our API offerings, please read the Tech sheet on Nilotinib Hydrochloride by filling in the contact form below.
Explore other Whitepapers:
Know More- Email us: [email protected]
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper


