Our Team at CPHI China 2025
About Dr.Reddy’s API Business
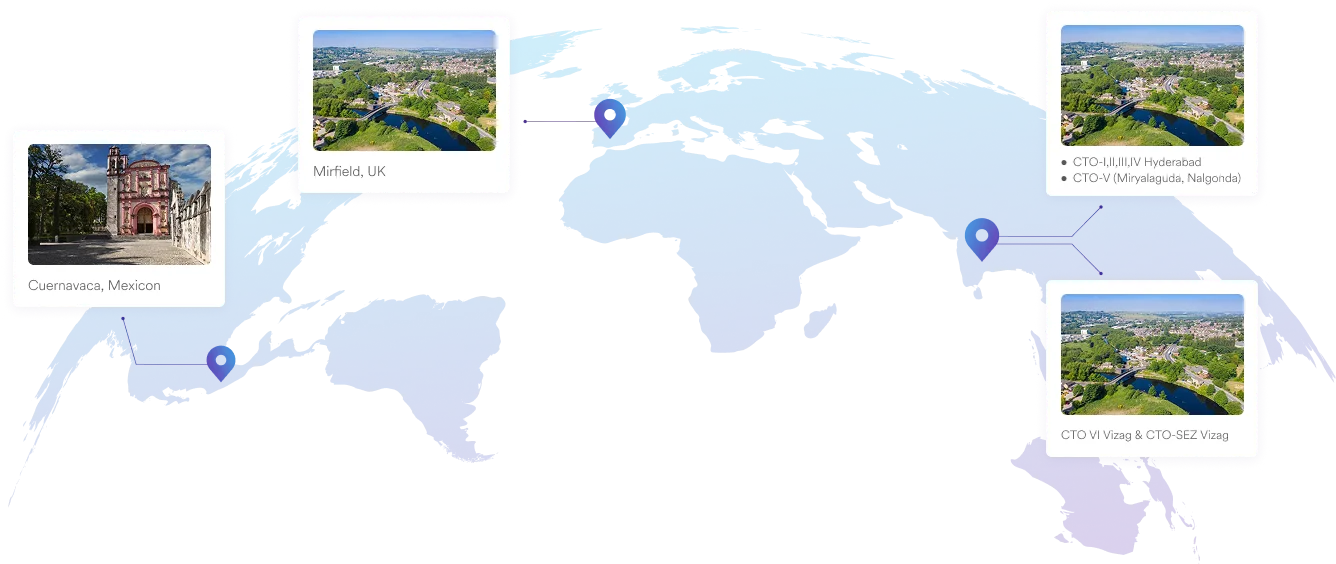
We specialise in manufacturing and supplying a wide range of APIs for generic formulation manufacturers in India and overseas. The company has a diversified portfolio of 250+ active pharmaceutical ingredients, which are used in major therapeutic areas such as gastrointestinal, cardiovascular, diabetology, oncology, pain management, and dermatology. Our API manufacturing facilities adhere to cGMP (ICH Q7) and are inspected on a regular basis by international regulatory bodies. We have eight commercially inspected USFDA production plants, six in India and one each in Mexico and the United Kingdom.
Dr. Reddy's API business operates in a number of global markets, including the United States of America, Latin America, Europe, India, Russia, and other CIS nations.

250+ APIs

8 Manufacturing sites

1970+ drug master files across global markets.

Over 325 ANDAs

Filed4500+ patents globally, out of which 1125 have been granted.

Experience with complex APIs and formulations supporting early market entries.

Preferred pharma API supplier for pharma companies in more than 80 countries.


Sustainability
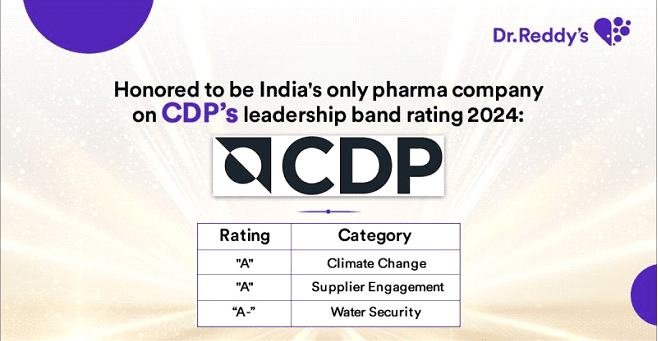
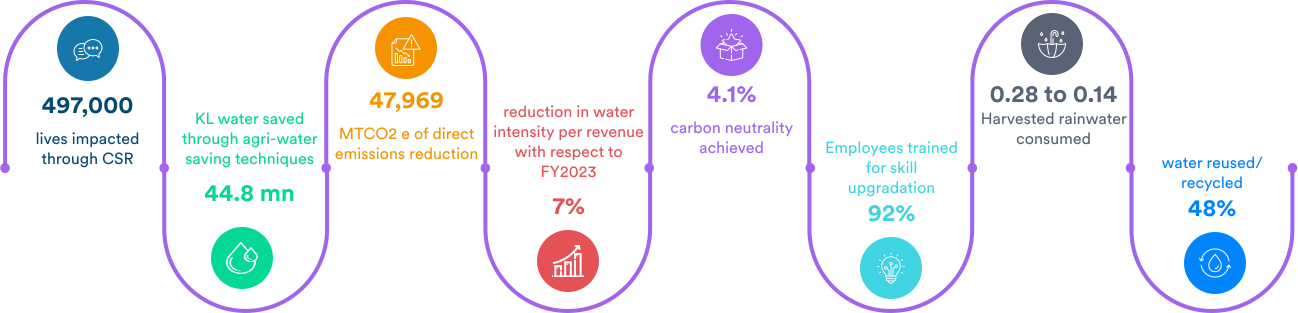
Dr. Reddy's is among the top sustainable pharmaceutical companies globally. We are listed in the sustainability yearbook of S&P 2022 for the second year in a row and the Bloomberg Gender-Equality Index (GEI) for the fifth consecutive year. In addition, we were ranked ninth in the Dow Jones Sustainability Index (DJSI) 2021 among the most sustainable pharmaceutical companies in the world. In our sustainability journey, these accolades are humbling and demonstrate that we're on the right track. These awards recognize our consistent performance in the environment, social, and governance (ESG) framework. Sustainability and ESG are becoming increasingly important topics for all stakeholders, so we will strive to maintain visibility in these areas.
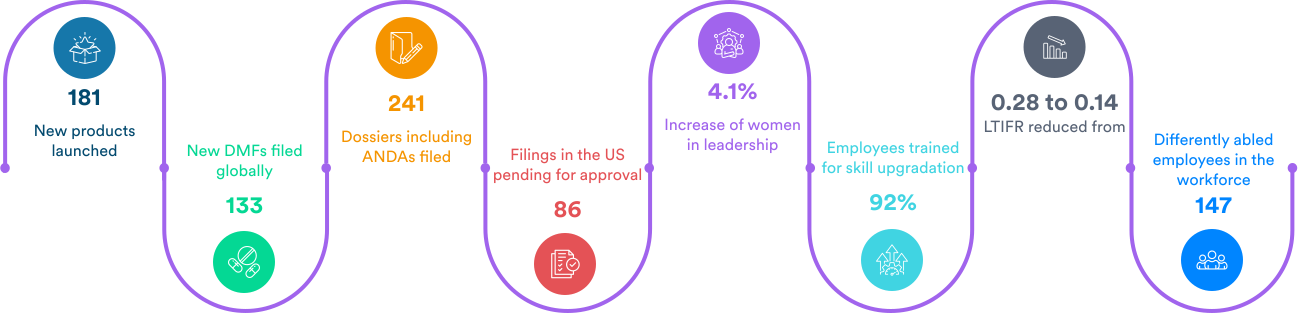
What we achieved in the past year:
Our Plants

About CPHI China 2025
This premier event brings together over 3,500+ exhibiting companies, 90,000+ pharma professionals, and the latest innovations across pharmaceutical ingredients, contract manufacturing, biopharma, packaging, drug delivery systems, and more. It's where key industry players meet to explore new opportunities, deepen existing partnerships, and stay ahead of fast-evolving market demands.
As part of this dynamic ecosystem, we’re looking forward to engaging with peers, partners, and potential collaborators. Whether you're looking to streamline your API sourcing, improve supply chain efficiency, or explore strategic synergies—we’re open to conversations that create value.
If you're attending CPHI China, we’d love to connect.Let’s discuss how we can support your growth with smarter, simpler, and future-ready sourcing solutions.