Regulatory Affairs: Global Expertise, Proven Compliance
Dr. Reddy’s offers unmatched regulatory expertise across international markets, ensuring our APIs meet the most stringent global standards. Our Regulatory Affairs (RA) team, with over 50 specialists, actively monitors evolving guidelines and proactively manages risk to support smooth approvals for complex APIs.
Global Reach & Proven Track Record
- DMFs filed across the U.S., Europe, Russia, Canada, China, Japan, Brazil, and more
- Expertise in DMF, CMC, IND, and NDA filings
- Strong partnerships with specialized law firms across geographies
- Facilities regularly audited by USFDA, EMA, WHO GMP, PMDA, KFDA, ANVISA, Health Canada, and others
Our regulatory strength ensures faster market access and reliable compliance for our global partners.

Experts Dedicated Team
State-of-the-art Manufacturing facilities
Total DMF(Drug Master Files) across global markets
Active DMFs
Our Global Network
- USA353
- Canada119
- Europe (ASMF)181
- Europe (CEP Filed)126
- Australia99
- New Zealand63
- Brazil174
- China115
- Israel35
- Japan55
- Korea115
- Russia54
- Saudi Arabia80
- Taiwan51
- RoW411
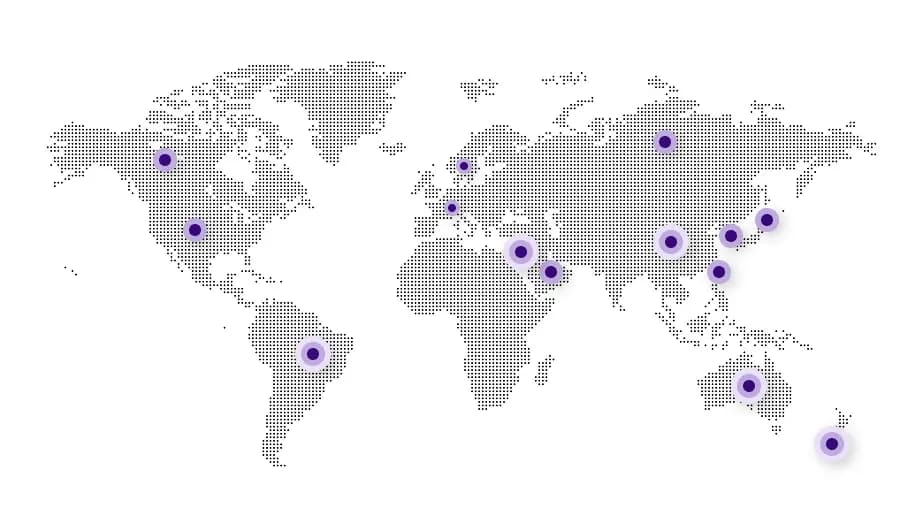
* As of Feb 2026
We understand the local market with broad market research & delivers high-quality regional specific DMFs to meet the regulatory requirements. Dr. Reddy's API business thrives on the deep technical strengths set up over the last 40+ years in consistently delivering high-quality APIs and manufacturing complex APIs. We follow best practices to improve our DMFs to match with the latest FDA regulatory standards. In addition, our expert Regulatory affairs & Quality teams follow the highest global standards of quality and regulatory compliance.
免責事項
このウェブサイト上のいかなる情報も、製品またはサービスへの言及を含め、販売の申し出を構成するものではなく、販売の申し出を表すものと解釈されるものでもありません。有効な特許により保護されている製品は、商用目的で提供または提供されるものではありません。ただし、特定のケースでは、Dr. Reddy の独自の裁量により、現地の法的要件に従って、そのような製品の研究用数量が、規制免除が存在する場所に、インド特許法第 107A 条 (Bolar 免除) に基づく規制提出の目的で提供される場合があります。購入者は、それぞれの市場における特許シナリオを含む製品またはサービスについて独自の評価を行う必要があり、すべての特許関連法的責任を負うことになります。Dr. Reddy は、商品性、特定目的への適合性、および非侵害の保証を含むがこれらに限定されない、明示または黙示を問わずすべての保証を否認します。

