Product Alert – Mavacamten
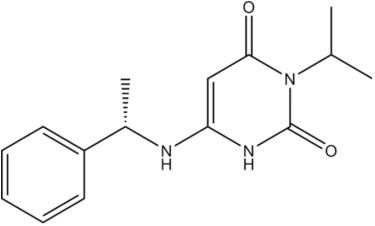
CAS No. 1642288-47-8
Mavacamten is an allosteric and reversible inhibitor selective for cardiac myosin. Mavacamten modulates the number of myosin heads that can enter “on actin” (power-generating) states, thus reducing the probability of force-producing (systolic) and residual (diastolic) cross-bridge formation. Excess myosin actin cross-bridge formation and dysregulation of the super-relaxed state are mechanistic hallmarks of HCM. Mavacamten shifts the overall myosin population towards an energy-sparing, recruitable, super-relaxed state. In HCM patients, myosin inhibition with Mavacamten reduces dynamic LVOT obstruction and improves cardiac filling pressures.
Dr. Reddy's API Offering*:
- Potential NCE-1 filing opportunity.
- Crystalline Form A (Validated).
- USDMF filed and DMF's planned for all other major markets
- Planning adequate capacity with a short lead time of 90 days.
- Regulatory compliance and quality ensure our API is genotoxic and nitrosamine-free, adhering to ICH M7 guidelines.
- Reliable suppliers for KSMs to ensure timely deliveries and strict adherence to specifications.
To learn more about our API offerings, please read the tech sheet on Mavacamten API by filling out the contact form below.
Explore other Whitepapers:
Know More- Email us: [email protected]
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper

