Tech Sheet on Enzalutamide Premix
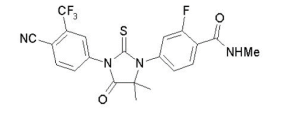
Chemical Name:
4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N- methylbenzamide.
Dr. Reddy's API Offerings*:
- Innovator has filed an Enzalutamide premix amorphous form manufactured through spray drying, which uses API and HPMCAS as excipients. We offer an Enzalutamide premix amorphous form with a non- infringing process and composition.
- Dr. Reddy's has a dedicated new manufacturing facility that offers Enzalutamide premix.
- Dr. Reddy's is among the earliest generic API manufacturers globally to file the USDMF for Enzalutamide API.
- Besides USDMF, country-specific regulatory filings are necessary for global market expansion, so we have diversified our filling to all the major regulated markets.
To learn more, download the Tech sheet on Enzalutamide Premix API HERE.
Should you have any specific queries? I would be happy to schedule a meeting at your earliest convenience.
*Note: Products under patent(s) are offered only for R&D purposes U/S 107A of the Patent Act and not for commercial sale.
Explore other Whitepapers:
Know More- Email us: [email protected]
-
 +91 40 49002222
+91 40 49002222
Download Now
Please fill the Contact form below in order to view the white paper


