Product Alert – Vonoprazan Fumarate
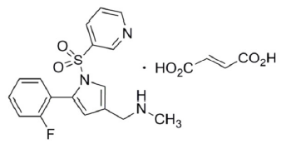
Dr. Reddy's Offerings:
- USDMF filed in februray 2024
- Offers crystalline innovator form.
Our API adheres to ICH M7 guidelines for genotoxic impurity (GTI) profiles, and we are actively evaluating strategies for nitrosamine impurities. We have also employed a robust process to achieve the consistent polymorph (innovator form) and morphology to ensure the desired in-vivo performance.
Our cGMP API manufacturing facility has undergone a thorough inspection by international regulatory authorities, and we have reliable suppliers for key starting materials to ensure timely deliveries and adherence to strict specifications. Moreover, we are equipped to provide sufficient capacity to supply development quantities within a short lead time of 90-120 days.
To learn more about our API offerings, please read the product alert on Vonoprazan API by filling out the contact form below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Complete el formulario de contacto a continuación para ver el documento técnico

