Tech Sheet - Cabozantinib (S)-malate API
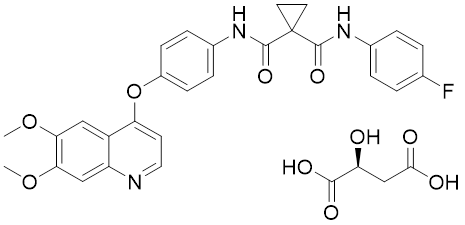
Cabozantinib S-malate is an orally bioavailable, small molecule receptor tyrosine kinase (RTK) inhibitor with potential antineoplastic activity.
Dr. Reddy's API Offering*
- We offer Cabozantinib S-malate – Crystalline form N2 (Innovator form).
- Cabozantinib HCl is an alternate salt providing 505(b)(2) opportunities in the US and early launch opportunities in Europe, Brazil, Turkey, Mexico, and South Korea.
- Dr. Reddy’s proposed synthetic process is IP-compliant.
- We employ a quality-by-design (QbD) approach for efficient processes and high-quality output.
- Effective control strategy for avoiding potential genotoxic and carcinogenic impurities
- Assured process safety and scalability.
- A short lead time of 120-180 days from the purchase order date (PO).
To learn more about our API offerings, please read the Tech sheet on Cabozantinib API by filling out the contact form below.
Note: *Products under patent(s) are offered only for R&D purposes U/S 107A of the Patent Act and not for commercial sale.
Explore other Whitepapers:
Know More- Email us: [email protected]
-
 +91 40 49002222
+91 40 49002222
Download Now
Complete el formulario de contacto a continuación para ver el documento técnico


