Substantially Pure Carfilzomib Amorphous for Generic Launch
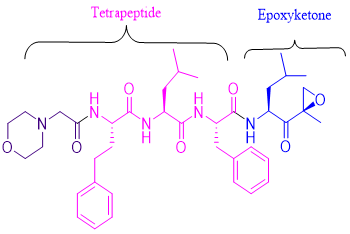
Kyprolis (carfilzomib) is a proteasome inhibitor that irreversibly binds to the N-terminal threonine-containing active sites of the 20S proteasome, the proteolytic core particle within the 26S proteasome. Carfilzomib had antiproliferative and proapoptotic activities in vitro in solid and hematologic tumour cells. In animals, carfilzomib inhibited proteasome activity in blood and tissue and delayed tumour growth.
Dr. Reddy's API Offering
Dr. Reddy’s Carfilzomib API is a notable example of the technical capabilities of product development. It offers our customers access to a generic API that shows distinct features of having optimal consumption co-efficient in line with the green chemistry concept and superlative quality resulting from a robust process at scale.
- We manufacture Carfilzomib amorphous API at our cGMP API manufacturing facility, successfully inspected by international regulatory authorities - USFDA, KFDA, WHO- GMP, Russian Federation, and ANVISA (document-based inspection completed and certificate is available).
- To achieve high supply assurance, our key starting materials (KSM) are sourced domestically, and one is from China.
- Continuous improvement is in place to achieve quality and supply excellence.
- The current batch size is about 1.5 kg (Per customer requirement, discrete lots can be supplied).
To learn more about our API offerings, please read the product alert Carfilzomib API by filling out the contact form below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
Complete el formulario de contacto a continuación para ver el documento técnico


