
Dec 13, 2025
Spray Drying: Advancing Pharmaceutical Performance Through Precision Engineering

Nov 16, 2025
Understanding DMF‑Quality APIs and Their Key Applications in Global Pharma

Oct 12, 2025
API Process Development: Turning Molecules into Manufacturable, Scalable, High-Quality APIs

Sep 9, 2025
Zero Liquid Discharge (ZLD) in Pharma API Manufacturing

Aug 14, 2025
FDA GMP Compliance for APIs Documentation, Validation, and Quality Systems

Jul 25, 2025
Development of Niche Products at Dr. Reddys API

Jun 6, 2025
Harnessing India's New Drug Development Capabilities for Global Impact

May 6, 2025
The Impact of Global Regulations on the Active Pharmaceutical Ingredient Industry

Apr 8, 2025
The Importance of Supply Chain Management and Dr. Reddy’s Excellence

Mar 5, 2025
Current Landscape of Cancer Treatment in India and Dr. Reddy's Role in Enhancing Care

Feb 10, 2025
Global Development Strategy for Generic Formulations and APIs

Jan 6, 2025
APIs for Emerging Markets: Opportunities and Challenges
Dec 15, 2024
Formulation Strategies and Pharmaceutical Analysis for Anti-diabetic APIs

Dec 2, 2024
API Quality Control Technologies & Strategies: Current Advancements for Benefiting Pharma Companies

Oct 9, 2024
A year of growth & Sustainability
Aug 30, 2024
Understanding Nitrosamines and GTI Issues in APIs: A Comprehensive Overview with Dr. Reddy's Perspective

Aug 28, 2024
Innovative Approaches to API Production in 2024: Shaping the Future of Pharma Companies

Jul 30, 2024
Continuous Manufacturing Process and Its Impact on Pharma Manufacturing

Jun 28, 2024
A Comparative Study on Naproxen Formulations

Jun 18, 2024
An effective strategy for the development of docetaxel
May 22, 2024
Preformulation Studies for Generic Omeprazole

May 15, 2024
A comparative study on enzalutamide Formulations

Apr 30, 2024
Navigating the Heart of Pharmaceuticals: An In-Depth Exploration of Active Pharmaceutical Ingredient Manufacturing for Essential Medicines
Apr 1, 2024
Presence of Organic Impurities in active pharmaceutical ingredients: An Overview

Mar 30, 2024
Strategies and Technologies for Enhancing Cost-Effectiveness in Active Pharmaceutical Ingredient (API) Production

Mar 29, 2024
Effective Formulation Development Strategies for Poorly Soluble Active Pharmaceutical Ingredients (APIs)

Feb 29, 2024
Ensuring Drug Safety: Comprehensive Analysis of Active Pharmaceutical Ingredient Lists
Feb 28, 2024
Unravelling the Science: Pharmaceutical Compositions and Process of Levetiracetam API

Nov 16, 2023
Impact of Venetoclax Exposure on Clinical Efficacy and Safety in Patients with Refractory Chronic Lymphocytic Leukemia

Aug 10, 2023
Demonstrating Equivalence of Generic Glatiramer Acetate: Ensuring Quality and Safety
May 25, 2023
Streamlining the Process: Preparing a Pharmaceutical Composition of Fosaprepitant API
Dec 30, 2022
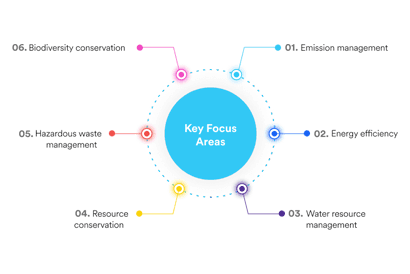
Steps taken by Dr. Reddy's Laboratories in controlling the environmental risks and safety management in the manufacturing of APIs
Dec 7, 2022
Meeting the New Regulatory Requirements and Controlling of Nitrosamine Impurities in Sartan API Production

Sep 14, 2022
Effective Formulation Development Strategies for Poorly Soluble Active Pharmaceutical Ingredients
Sep 13, 2022
Reshaping the Pharmaceutical API Industry with Digital Transformation

Aug 2, 2022
7 Ways how Active Pharmaceutical Ingredients (APIs) Enable Indian Pharmaceutical Companies to Grow

Aug 2, 2022
How is the Indian Pharmaceutical industry gearing up for 2022-2023?
Apr 14, 2022
Impact of COVID-19 on Indian HPAPI industry
Apr 14, 2022
The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry
Sep 14, 2021
瑞迪博士的61种原料药均采用巴西卫生监督局(ANVISA)认证的良好生产规范(GMP) 药品成分
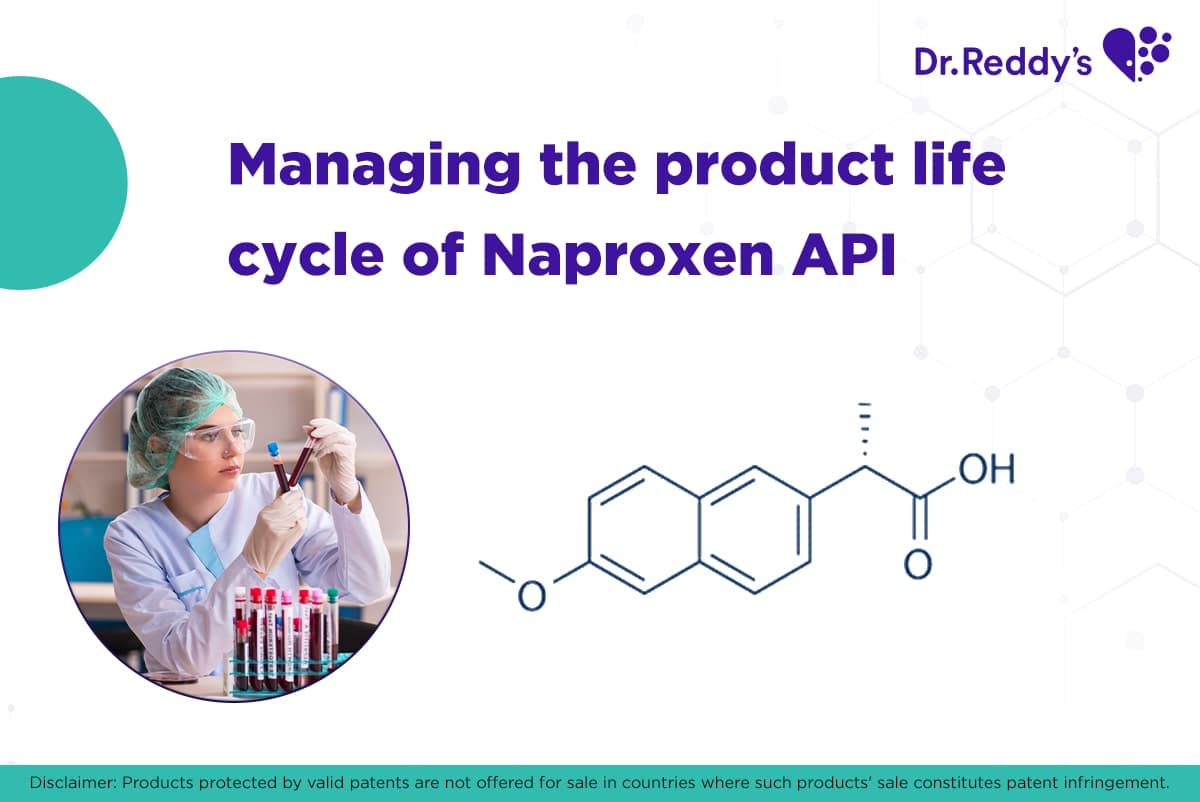
Jul 7, 2021
产品生命周期管理:萘普生(Naproxen)原料药和配方

May 27, 2021
了解原料药: 拉马尼·苏萨拉 (Ramani Susarla)博士的制药原料展览会(CPHI)演讲
Mar 31, 2021
EMA Nitrosamine Guidance – Deadline March 31, 2021

Nov 26, 2020
2021年患者为中心和供应链安全 - 拉杰什·萨达南丹 (Rajesh Sadanandan)就2021年世界医药原料展(CPhI)制药趋势发表了讲话

Nov 19, 2020
瑞迪博士关于在单颗粒水平上同时进行自动图像分析和拉曼光谱的研究出版物

Sep 14, 2020
范式转变将持续 - 索里•古德拉瓦莱蒂(Sauri Gudlavalleti)与CME经理谈论新冠病毒病应对措施

Sep 7, 2020
充满挑战与机遇的一年 - 制药服务和活性成分(PSAI)全球主管迪帕克•萨普拉接受了《药物厂商》(The Medicine Maker)的采访

Sep 2, 2020
新冠病毒病和原料药采购的下一步是什么?

Aug 25, 2020
印度医疗改革提供了蓬勃发展的医药市场机会: 采访迪帕克·萨普拉(Deepak Sapra)

Jun 1, 2020
瑞迪博士正在通过大规模生产能力和后向一体化来改善普瑞巴林(Pregabalin)原料药的供应链绩效

Dec 1, 2019
Enhancing Supply Chain Security for Levetiracetam API
免责声明
本網站上的任何信息,包括對任何產品或服務的任何提及,均不構成銷售要約或被解釋為代表銷售要約。受有效專利保護的產品不得提供或供應用於商業用途。但是,在某些情況下,雷迪博士可自行決定並根據當地法律要求,在存在此類監管豁免的任何地方,提供此類產品的研究數量,以根據《印度專利法》第 107A 條(Bolar 豁免)進行監管提交。購買者應對其各自市場的產品或服務(包括專利情況)進行獨立評估,並對所有與專利相關的責任負責。 Dr. Reddy's 不承擔任何明示或暗示的保證,包括但不限於適銷性、適用於特定用途和非侵權的保證。





