Product Alert - Relugolix API
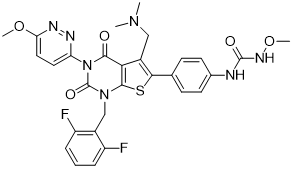
CAS No: 737789-87-6
As an early mover in API development, we are committed to regulatory compliance, have DMFs filling in the US and Brazil, and plan to file the DMFs in significant markets. Our API is available in an anhydrous crystalline form (the innovator form), which can be provided in customized sizes based on your requirements. We also offer a finished dosage form of Relugolix in selected markets.
Our synthetic process ensures consistent polymorph and amorphous forms for optimal in-vivo performance. Additionally, our API adheres to ICH M7 guidelines for genotoxic impurity (GTI) profiles, and we are actively evaluating strategies for nitrosamine impurities.
To learn more about our API offerings, please read the product alert on Relugolix API by filling out the contact form below.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
请填写下面的联系表以查看白皮书

