Tech sheet – Apremilast
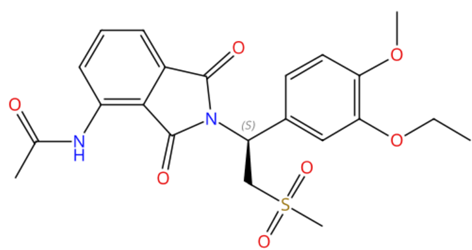
Dr. Reddy's API Offerings*:
- Dr. Reddy's is among the earliest generic API manufacturers globally for Apremilast API and filed the USDMF in September 2016 (Form-B) and June 2017 (Amorphous). Besides this, we have DMF filings in Canada and Brazil.
- Offers the same form as the innovator drug, facilitating successful bioequivalence studies.
Manufacturing and Capacity:
We manufacture Apremilast at our cGMP API manufacturing facility in Vizag (CTO-SEZ), which is successfully inspected by global regulatory authorities, including the US FDA, and is EU GMP certified too. We have sufficient capacity to supply the commercial quantities in a short lead time.
To learn more, download the techsheet on Apremilast API HERE.
Should you have any specific queries? I would be happy to schedule a meeting at your earliest convenience.
*Note: Products under patent(s) are offered only for R&D purposes U/S 107A of the Patent Act and not for commercial sale.
Explore other Whitepapers:
Know More- Email us: api@drreddys.com
-
 +91 40 49002222
+91 40 49002222
Download Now
请填写下面的联系表以查看白皮书


