
Oct 10, 2025
A Bold Leap for Global HIV Prevention: Lenacapavir Agreement Signed

Aug 5, 2025
Tentative Approval for Generic Lumateperone capsules
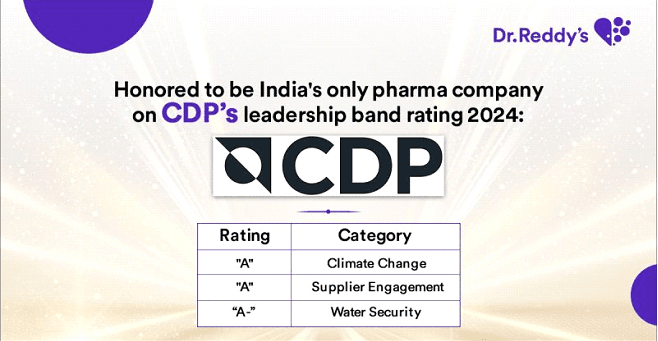
Jul 10, 2025
CDP Recognition

Jun 24, 2025
Dr. Reddys API team at CPHI China 2025

Apr 9, 2025
CPHI Japan 2025 - Exhibitor showcase session by Nirav K Shah, Vice President, Head API, North Asia

Apr 9, 2025
Dr. Reddys API team at CPHI Japan 2025
Mar 17, 2025
Dr. Reddys API team at DCAT 2025

Feb 25, 2025
Dr. Reddy’s Wins Big at “Express Pharma Excellence Awards 2025”

Dec 18, 2024
Celebrating Creativity and Festivity: API Calendar Launch 2025!

Nov 29, 2024
Dr. Reddy’s wins CPHI & PMEC India Award for Excellence in Ancillary Pharma Services - Supply Chain & Logistics for enhancing customer experience

Nov 29, 2024
Dr. Reddy’s wins CPHI & PMEC India Award for developing a continuous API manufacturing process.

Nov 29, 2024
Dr. Reddy's API team at CPHI India 2024

Oct 13, 2024
Dr. Reddy's API team at CPHI Milan 2024

Sep 27, 2024
Gunjan Singh at the CPHI Webinar: The Changing Dynamics of Global API Manufacturing

Sep 27, 2024
Karun Gaur at CPHI Milan: Panel Discussion on Pharma Manufacturing in Emerging Markets - Moving Towards Localization in Africa

Jul 8, 2024
Deepak Sapra's Visionary Keynote on Healthcare Innovation at DXEM 2024

May 8, 2024
Dr. Reddy's AI Day: Igniting the Future of Healthcare with Innovation

Apr 25, 2024
USP recognized Dr. Reddy’s with a crystal award for significant contribution towards “New and modernized monograph submissions”

Apr 22, 2024
Dr. Reddy’s API at CPHI Japan 2024

Apr 5, 2024
Dr. Reddys Strategic collaboration with Industrial Promotion Services (IPS) to support the East African Combined Pharmaceutical Center (EACPC) in Kenya

Jan 2, 2024
Dr. Reddy's API Calendar 2024 Unveiling: A Magical Celebration of Creativity, Joy, and Togetherness with Our Global Team's Talented Young Artists

Dec 5, 2023
Dr. Reddy's Pioneers Pharma Advancements Through Strategic Partnerships and Innovation: A Glimpse into Vision 2030: Growth Trajectory and Opportunities for Indian Pharma

Nov 30, 2023
Dr. Reddy’s at CPHI India 2023

Nov 23, 2023
Dr. Reddy’s at 5th CII LIFE SCIENCES SUMMIT 2023 - Making India a Global Pharma and Lifesciences Innovation Hub

Nov 11, 2023
Asia-Pacific Climate Leader Award

Nov 10, 2023
Dr. Reddy’s Achieves AEO-T3 Certification, Elevating Global Trade Facilitation

Oct 28, 2023
Dr. Reddy’s API at CPHI Worldwide 2023

Aug 20, 2023
Dr. Reddys at G20 Summit 2023

Jun 25, 2023
Dr. Reddy’s API at CPHI China 2023

Mar 15, 2023
Being Prepared for What's Next and a Shared Purpose of Serving Patients Whatever it Takes

Feb 15, 2023
Dr. Reddy’s included in Bloomberg Gender-Equality Index for the 6th year in a row and S&P Global’s Sustainability Yearbook for the 3rd year

Jan 5, 2023
Dr. Reddy's API calendar 2023

Nov 11, 2022
COVID-19: Prime Minister of India Meets Pharma leaders

Nov 6, 2022
Addressing NDMAs in Pharmaceuticals
Nov 5, 2022
Dr.Reddy's at CPHI India 2022 Panel Discussion

Nov 4, 2022
Dr. Reddy’s API received the excellence in API – Technical Documentation award at Aché Laboratórios Parcerias para a Excelência (Partnerships – Excellence) event!

Oct 6, 2022
Dr. Reddy's API participated in the 2022 Pharmaceutical Industry and Regulators Symposium at ANVISA
Sep 27, 2022
Expert statement by Srividya Ramakrishnan, Head, API process engineering, Dr. Reddy’s Laboratories. Published in CHE Manager

Sep 25, 2022
Dr. Reddys attended ACHEMA 2022 Conference

Nov 11, 2021
Dr. Reddy’s API team is a 2021 winner in – API Supplier of the year at Global generics & Biosimilars awards

Nov 11, 2021
The winner of the Corporate social responsibility (CSR) Initiative of the year at Global generics & Biosimilars Awards

Oct 7, 2021
The winner of the Best Indian Company in the US - Manufacturing Sector for the year 2021
Oct 6, 2021
Sugammadex Sodium API – CADIFA Approval by ANVISA

May 27, 2021
Deepak Sapra habla acerca del fármaco 2 DG anti-COVID de la DRDO

May 27, 2021
Dr.Reddy’s gearing up with Covid portfolio: Deepak Sapra, CEO of API & Services, speaks to India Today

Jan 21, 2021
El Mejor Equipo de Cadena de Suministros del Año - Pharma - 2021

Nov 10, 2020
Anil Patni de Dr. Reddy’s Laboratories hizo una presentación acerca de los HPAPI (Ingredientes Farmacéuticos Activos Altamente Potentes) en Manufacturing Chemist Live Virtual

Nov 3, 2020
Dr. Reddy’s Laboratories wins “API Supplier of the Year” award at the Global Generics & Biosimilars Awards 2020

Oct 19, 2020
Se reconoció a Dr. Reddy’s en los CPhI Pharma Awards 2020 como ganador en la categoría de “Excelencia en Farmacéutica:

Oct 13, 2020
Mesa de Debate del Festival CPhI de Pharma: Abordaje de la Contaminación de Nitrosamina

Oct 5, 2020
Dr. Reddy’s Laboratories joins Science Based Targets initiative (SBTi) and sets 2030 GHG emission targets

Oct 5, 2020
CPhI Festival of Pharma Panel discussion:Innovation across Borders: How Pharma and innovators are fighting back against COVID-19 and ways forward.

Jul 1, 2020
Socios de Dr. Reddy’s con FUJIFILM y Global Response Aid por Avigan® (favipiravir), un posible tratamiento para la COVID-19

Apr 14, 2020
Declaración de Dr. Reddy’s sobre la pandemia de COVID-19

Apr 1, 2020
Dr. Reddy's aparece en un artículo de Global Pharma Insights sobre "Contaminación por nitrosamina: reevaluación de la cadena de suministro de la industria farmacéutica"

Apr 1, 2020
Certificado de Sustentabilidad (CEP) otorgado por el Levofloxacino Hemihidrato.

Mar 1, 2020
DMF de Estados Unidos para Apalutamide – Ahora Disponible

Mar 1, 2020
Informe de Sustentabilidad 2018-19 de Dr. Reddy's

Dec 1, 2019
Dr. Reddy's API Team moves into its new office in China

Nov 1, 2019
Dr. Reddy’s Laboratories gana el premio al “Proveedor de IFA del Año” en los Global Generics & Biosimilars Awards de 2019
Descargo de responsabilidad
Ninguna información en este sitio web, incluyendo cualquier referencia a cualquier producto o servicio, constituye una oferta de venta ni se interpretará como tal. Los productos protegidos por patentes válidas no se ofrecen ni suministran para uso comercial. Sin embargo, en ciertos casos, a discreción exclusiva de Dr. Reddy's y sujeto a los requisitos legales locales, las cantidades de investigación de dichos productos pueden ofrecerse para fines de presentaciones regulatorias según la Sección 107A de la Ley de Patentes de la India (exención de Bolar), donde existan dichas exenciones regulatorias. Los compradores deben realizar su propia evaluación del producto o servicio, incluyendo el escenario de patentes en sus respectivos mercados, y serán responsables de todas las responsabilidades relacionadas con las patentes. Dr. Reddy's renuncia a todas las garantías, expresas o implícitas, incluyendo, entre otras, las garantías de comerciabilidad, idoneidad para un propósito particular y no infracción.





